Isolated Diffusion Restriction Preceding Contrast Enhancement in Glioblastoma Multiforme is Associated with Short-Term Survival
Jai Jai Shiva Shankar 1, *![]() , Adil Bata 2
, Adil Bata 2![]() , Namita Sinha 3
, Namita Sinha 3![]()
- Department of Radiology, Division of Neuroradiology, Dalhousie University, Halifax and University of Manitoba, Winnipeg, Canada
- Department of Radiology, Dalhousie University, QE II Health Sciences Centre, Winnipeg, Canada
- Department of Pathology, University of Manitoba, Winnipeg, Canada
* Correspondence: Jai Jai Shiva Shankar ![]()
Received: August 7, 2018 | Accepted: October 22, 2018 | Published: October 26, 2018
OBM Neurobiology 2018, Volume 2, Issue 4, doi:10.21926/obm.neurobiol.1804013
Academic Editor: Antonio Meola
Special Issue: Tumors of the Central Nervous System
Recommended citation: Shankar JJS, Bata A, Sinha N. Isolated Diffusion Restriction Preceding Contrast Enhancement in Glioblastoma Multiforme is Associated with Short-Term Survival. OBM Neurobiology 2018; 2(4): 013; doi:10.21926/obm.neurobiol.1804013.
© 2018 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
(1) Background: Current imaging standard for detecting and assessing glioblastoma multiforme (GBM) progression depends on contrast-enhancement on brain magnetic resonance imaging (MRI). Isolated foci of diffusion restriction have been observed to precede enhancement in GBM. The aim of our study was to investigate the frequency of isolated diffusion restriction that precede corresponding enhancement and to investigate the association between isolated diffusion restriction and survival in patients with GBM. (2) Methods: MRI of the brain, including diffusion weighted images (DWI) and apparent diffusion coefficient (ADC) maps were retrospectively examined in 102 consecutively treated patients with histopathologically confirmed GBM. Images were assessed for the presence of isolated diffusion restriction in GBM and identified lesions were monitored for enhancement on follow-up MRI. Data were collected on the length of time for enhancement to appear, normalized ADC, and overall survival of patients. (3) Results: Forty patients (39.2%) showed areas of isolated diffusion restriction. Ten patients (25%) developed corresponding enhancement on follow-up imaging after an average of 145 days after the index imaging. In patients with isolated restricted diffusion, the mean ADC was 721.4 ± 117.2 mm2/s compared to 888.7 ± 85.2 mm2/s in the normal appearing-white matter (NAWM) in contralateral hemisphere (p<0.001). On survival analysis, the overall survival was longer (p=0.036) in patients with isolated restricted diffusion and these patients had survival benefit (p=0.006) in the early follow-up period. (4) Conclusions: Isolated restricted diffusion in GBM precedes corresponding enhancement in a subset of patients with GBM and was associated with early survival benefit.
Keywords
Diffusion weighted imaging; glioblastoma multiforme; apparent diffusion coefficient
1. Introduction
Patients with glioblastoma multiforme (GBM) have poor prognosis with an overall survival time of less than twelve months despite aggressive treatment [1]. GBM’s highly variable response to existing therapies coupled with its short survival times [2], underlines the need for additional imaging biomarkers capable of identifying earlier signs of disease progression to augment treatment management and accelerate decision-making. The current imaging standards for detecting and assessing tumour progression in patients with GBM depend heavily on changes in contrast-enhancing abnormalities on brain magnetic resonance imaging (MRI) [3,4]. Contrast enhancement on gadolinium enhanced MRI is based on the immature leaky capillaries in the region of the GBM. Contrast enhancing abnormalities are examined and characterized to estimate prognosis and also to guide management of these malignant brain tumours [5].
Diffusion weighted imaging (DWI), a MRI method based on Brownian motion of water molecules, has become an important tool in the characterization of brain tumours [5,6]. In tumours, restricted diffusion is seen in areas showing very high cellularity [5,7,8]. The increased cellularity leads to the reduction of interstitial space resulting in restricted diffusion. Diffusion restriction is usually identified in GBM in the area of contrast enhancement. Gupta et al have shown that the increased cellularity associated with restricted diffusion can be seen before enhancement on T1-weighted (T1W) post gadolinium images [3]. The aim of our study was to investigate the frequency of isolated diffusion restriction that precede corresponding enhancement and to investigate any association between isolated diffusion restriction and survival in patients with GBM. The hypothesis of our study was that isolated diffusion restriction preceding enhancement is associated with survival in patients with GBM.
2. Methods
Patient selection: Consecutive patients over a 3-year period were identified from our institutional brain tumour database with confirmed diagnosis of GBM on biopsy or resection and their data were retrospectively analyzed. The study was approved by the institutional research ethics board. Patients with the following set of axial images on their first diagnostic MRI, before any medical or surgical treatment, were included in our study: 1) DWI; 2) ADC (apparent diffusion coefficient) maps; 3) axial post gadolinium T1W images; and 4) axial fluid-attenuating inversion recovery (FLAIR) images. Additional information on patients’ demographics as well as clinical, surgical, follow-up, and survival data was obtained from the brain tumour database. Karnofski performance score, which defined the functional status of the patient, was also obtained from the brain tumor database. All procedures performed in studies involving human participants were in accordance with the institutional ethical standards.
Image acquisition: MRI were performed on a 1.5 T magnet scanner (Singa, GE Healthcare) with the following tumor protocol: DWI, pre and post gadolinium T1W images, T2 and FLAIR image of the brain. The DWI were acquired using single-shot echo-planar imaging with 8000 ms TR, 73.6 ms TE, 260-mm FOV, 160x192 matrix size, 5- mm section thickness with 1.5 mm intersection gap, and 1000 and 0 mm 2 /s b-values obtained in 3 orthogonal directions. FLAIR images were acquired as fast spin echo images using 8000 ms TR, 120 ms TE, 2000 ms TI, 220-mm FOV, 256x254 matrix size, 5-mm section thickness with 1.5 mm intersection gap. T2 images were acquired as fast spin echo images using 8000 ms TR, 120 ms TE, 220-mm FOV, 256x254 matrix size, 5-mm section thickness with 1.5 mm intersection gap. Both pre and post-contrast T1W images were acquired as fast spin echo images using single-shot echo-planar imaging with 500 ms TR, 22.8 ms TE, 220-mm FOV, 320 x 192 matrix size, 5-mm section thickness with 1.5 mm intersection gap.
Image analysis: Regions with low ADC signifying restricted diffusion both within and outside of the contrast-enhanced tumour were identified by one of the coauthors (AB) under the guidance of a fellowship trained neuroradiologist (JS) with more than 7 years of experience as Neuroradiology staff. Restricted diffusion was defined as hyper-intensity on DWI and hypo-intensity on ADC map compared to the normal-appearing white matter (NAWM) in the contra-lateral hemisphere. These areas of restricted diffusion were first identified on visual inspection of the images and then by quantification on the ADC maps. Patients with regions of isolated restricted diffusions (defined as regions with low ADC without corresponding enhancement on the post-gadolinium T1W images) were identified (Figure 1). These regions of isolated restricted diffusion with no enhancement on first MRI, were monitored for enhancement on follow-up MRI. The final cohort of patients included those who demonstrated: 1) isolated restricted diffusion or diffusion restriction larger than enhancement and 2) no corresponding hemorrhage on their first MRI and those with no immediate postoperative changes in the region of isolated restricted diffusion, on their follow up MRI.
The degree of restriction was quantified as the lowest ADC value within each region of restricted diffusion. The degree of restriction was calculated by manually outlining the region of restricted diffusion (using ‘Freeform mark up’ tool) on the slice it was seen. ADC was also measured in the NAWM in the contra-lateral hemisphere using ‘Freeform mark up’ tool with an area of at least 10 mm2. This was used to calculate the normalised ADC (nADC, i.e., ratio of minimum ADC in the tumor to that in contralateral NAWM). The ADC values (in mm2/s) were recorded on a PACS work station and not on an MRI console. These two values have been shown to be comparable and not statistically different [9]. The total volume of enhancing component of the tumor was calculated by manually outlining the abnormality (using ‘Freeform mark up’ tool) on all the slices. The sum of the areas on each slice was multiplied by the slice thickness and the inter-slice gap to get the volume. Volume of tumour, time interval for enhancement to appear, and overall survival of patients from the time of first imaging were recorded.
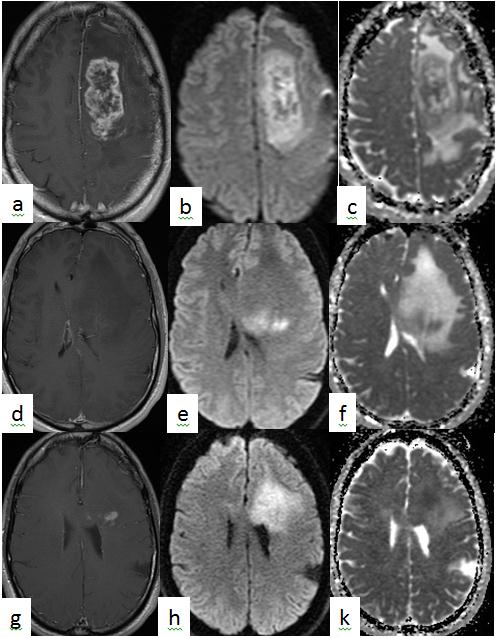
Figure 1 Patient with GBM with heterogeneously enhancing tumor on post-gadolinium T1 axial images (a) showing heterogeneous pattern of diffusion restriction on diffusion weighted images (DWI)(b) and apparent diffusion coefficient (ADC) map (c). Superior to the enhancing lesion, there was no enhancement on poste-gadolinium T1 weighted images (d) but with isolated restricted diffusion on DWI (e) and ADC map (f). On follow up 2.5 months later, enhancement (g) was seen on post-gadolinium T1 axial images, in the same area of isolated restricted diffusion on DWI (h) and ADC map (i).
Statistics- Descriptive statistics were performed. The two group of patients with and without isolated restricted diffusion were compared using Wilcoxon signed-rank test to analyze any significant difference. Kaplan Meier survival statistics were performed in Graph-pad statistical package using Log-rank and Gehan-Breslow-Wilcoxon tests to assess the difference in the survival pattern between the two groups. In comparison to the Log-rank test, the Gehan-Breslow-Wilcoxon test places more weight on deaths at early time points, and hence is more sensitive to the detection of early survival differences.
3. Results
Over 3 consecutive years, a total of 155 patients had a pathologically confirmed GBM in our institution. Of these, 102 patients fulfilled our imaging inclusion criteria. Other patients were excluded since they did not have DWI at the time of diagnostic MRI. Restricted diffusion within or adjacent to enhancing tumour was seen in 97 patients (95.1%) with 40 patients (39.2%) with isolated restricted diffusion (Table 1). Of these 40 patients with isolated restricted diffusion, 10 (25%) developed corresponding enhancement on follow-up examinations after an average of 144.75 ± 110.48 days from the index imaging. The longest time interval for enhancement on follow-up imaging was after 359 days in one patient. Another 10 of the 40 patients (25%) did not have follow-up MRI and another 4 patients (10%) did not have ADC maps on follow-up MRI. In 6 patients (15%), post-surgical changes interfered with analysis of DWI. In these patients with isolated restricted diffusion, we were not able to assess the follow-up enhancement. The remaining 10 patients (25%) had regions of isolated restricted diffusion that did not show corresponding enhancement on available follow-up imaging. In patients with isolated restricted diffusion, the mean ADC was 721.4 ± 117.2 mm2/s compared to 888.7 ± 85.2 mm2/s in the NAWM (p <0.001) (Figure 2).
Table 1 Demographic, imaging, treatment and survival characteristics of the GBM patients with and without isolated restricted diffusion.
|
|
With Isolated restriction diffusion (Mean ± SD) |
Without isolated restricted diffusion (Mean ± SD) |
P value |
|
N |
40 (41.2%) |
57 (58.8%) |
|
|
Age |
59.98 ± 10.38 |
62.8.10 ± 13.88 |
0.26 |
|
Sex (M/F) |
21/19 |
30/17 |
0.99 |
|
KPS at diagnosis |
76.58 ± 16.5 |
73.56 ± 14.95 |
0.48 |
|
Degree of resection (1/2/3) |
31.4/17.1/51.4 |
54.4/8.7/36.9 |
0.12 |
|
Volume of tumor (mm3) |
23048.91 ± 21789.92 |
29431.53 ± 21964.34 |
0.16 |
|
Survival (days) |
486 ± 363.5 |
291.9 ± 344.3 |
0.036 |
Note: N-number; M-Male; F-Female; SD-standard deviation; KPS-Karnofski performance score; Degree of resection: 1-biopsy; 2-partial resection; 3-gross total resection.
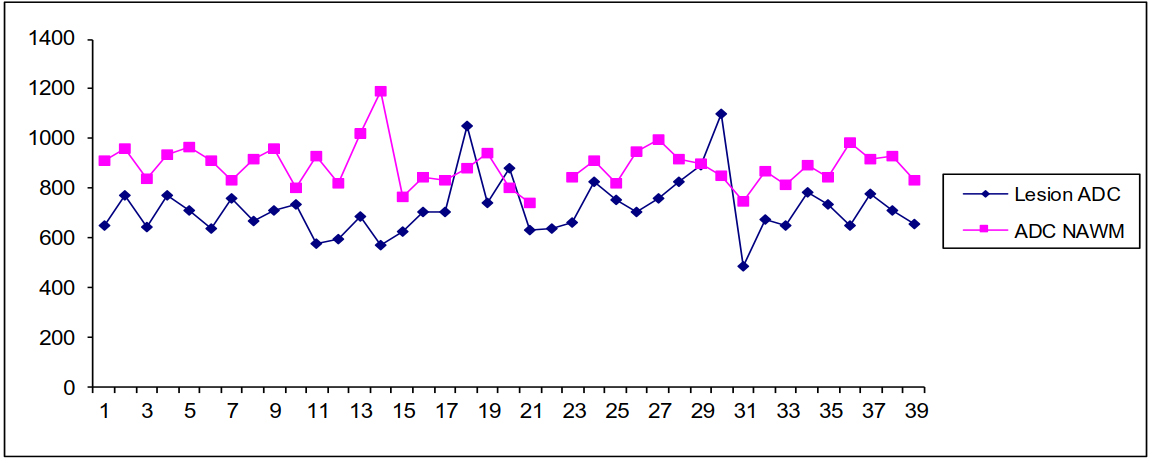
Figure 2 Apparent Diffusion Coefficient (ADC in mm2/sec) in the region of isolated restricted diffusion (shown as lesion ADC) compared to that in the normal appearing white matter (NAWM) in the contralateral hemisphere in 40 patients with glioblastoma multiforme.
We had clinical follow up for all patients and the overall survival was significantly longer (p=0.036) in patients with isolated restricted diffusion after index MRI than those without (Table 1). On survival analysis (Figure 3), the Gehan-Breslow-Wilcoxon test showed significant (p=0.006) survival benefit in the early follow-up period reflecting longer survival (median survival of 456 days vs 173 days) in patient with isolated diffusion restriction. However there was no significant difference (p=0.134) in the overall survival in these two groups of patients on Log-rank (Mantel-Cox) test.

Figure 3 Kaplan-Meir curve showing the early survival benefit in patients showing the isolated restricted diffusion (Gehan-Breslow-Wilcoxon test, p=0.006) but no overall survival benefit (Log-rank Mantel-Cox test, p=0.134). DWI- represent those with isolated restricted diffusion; No DWI- represent no evidence of isolated restricted diffusion.
Among patients with isolated restricted diffusion, those who developed enhancement on follow-up had a trend (p=0.059) towards higher average survival (474.1 ± 369.8 days) compared to those who did not progress to enhancement (729.3 ± 404.7 days). However the small numbers in each group did not allow for any meaningful analysis. The patients with isolated restricted diffusion progressing to enhancement (214.6 ± 120.2 days) had a trend towards shorter follow up (p= 0.059) compared to those who did not progress to enhancement (429.8 ± 308.9 days). Thus a shorter follow-up period was not responsible for the lack of corresponding enhancement.
4. Discussion
In patients with GBM, the regions of isolated restricted diffusion, on their diagnostic MRI, is likely a focus of tumour, even in the absence of enhancement. This is an important concept to consider since the current definition of a tumour on imaging only includes the contrast enhancing component of lesions (RANO criteria) [10]. Foci of isolated restricted diffusion at the time of imaging could be the focus of recurrent or residual tumor. It should also be noted that these foci of restricted diffusion could be seen long before corresponding contrast enhancement and could be separate from the enhancing component in patients with new or recurrent GBM. Earlier detection of these unrecognized foci of GBM could lead to more timely and suitable treatment that may improve prognosis.
In our study, isolated restricted diffusion was identified in 39.2% of GBM patients and in more than 20% of them restricted diffusion preceded corresponding enhancement. On an average, enhancement appeared five months after detection of isolated restricted diffusion on the diagnostic MRI. Gupta et al found isolated restricted diffusion in a similar proportion (40.3%) of their patients (27 out of 67). They found enhancement at the site of restricted diffusion in 85.2% patient after a median of 3.0 months [3]. By recognizing these foci of isolated restricted diffusion on diagnostic MRI, as potential foci of tumour, a significant delay can be avoided in targeted treatment.
Survival data showed that isolated restricted diffusion was associated with longer short-term survival. We hypothesize that the patients with GBM showing isolated restricted diffusion may have different molecular signatures compared to those without. This hypothesis remains to be tested as we did not have molecular signature available in these patient for analysis. We plan to verify this in a prospective study. The patients with isolated restricted diffusion progressing to enhancement had a trend towards shorter follow up (p= 0.059) compared to those who did not progress to enhancement. This suggested that a shorter follow-up period was not responsible for the lack of corresponding enhancement. This may also suggest that progression to enhancement was a poor prognostic factor in terms of overall survival. However the number of patients in these subgroups was small to reach any meaningful conclusion.
Diffusion imaging is a part of routine brain tumor MRI. Our findings highlighted the added value of isolated restricted diffusion without corresponding enhancement on the diagnostic MRI, in patients with GBM. Recognizing these areas of restricted diffusion may provide a more comprehensive approach to the diagnosis and treatment monitoring of GBM without any additional resources. Moreover, inclusion of isolated restricted diffusion in treatment planning of GBM, both on surgery or subsequent radiotherapy, may result in more predictable patient outcomes. Investigators continue to assess the role of isolated restricted diffusion as a predictor of GBM progression and prognosis [11]. Recognizing isolated diffusion restriction on post-treatment follow-up MRI may also be useful to gauge treatment efficacy and to identify recurrent or residual foci even in the absence of corresponding enhancement. For example, tumors treated with anti-angiogenic agents (e.g. bevacizumab) have been reported to reduce contrast enhancement, resulting in an overestimation of treatment response on conventional imaging [12]. Nevertheless, this interesting and important imaging phenomenon in GBM still requires rigorous clinical validation, which warrants further studies in larger patient populations.
Limitations: This study was limited in terms of its small number of patients and its retrospective nature. Our study was also limited due to the lack of data on the molecular markers and inconsistent follow up imaging in these patients. However our study generates an important hypothesis in sequential patients that remains to be tested in a larger prospective study. The study was also limited by the missing imaging data points in 14 patients. Of these, 10 patients died and did not have any follow up images. Another 4 patients had follow up images but did not have DWI done on follow up. The 10 patients who died on follow up were censored. The remaining 4 out of the 40 patients (10%) did not have diffusion images on follow up. These patients did not give any information on the length of time for appearance of enhancement. These patients were still included on survival analysis as we had clinical information on survival in all of them.
5. Conclusions
Isolated restricted diffusion preceded the corresponding enhancement in a subset of patients with GBM and was associated with early survival benefit. Recognition of this important imaging phenomenon in GBM may foster its uptake into routine treatment planning, which may enable more predictable outcomes in the future. Prospective study in larger patient cohort is warranted to confirm our observations.
Author Contributions
Adil Bata- collected the data and wrote the first draft.
Namita Sinha- Intellectual contribution to the manuscript and editing the final draft.
Jai Shankar- Original idea, data analysis, final manuscript editing and submission.
Funding
This work was kindly supported by Summer Student grant (5000) from Radiology Research Foundation, Dalhousie University, Halifax.
Competing Interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Salcman M. Glioblastoma multiforme and anaplastic astrocytoma. In: Kaye AH, Laws ER, editors. Brain tumors: an encyclopedic approach. 2nd ed. London: Churchill Livingstone; 2001.
- Herbert C, Williams M, Sawyer H, Greenslade M, Cornes P, Hopkins K. Treatment of glioblastoma multiforme with radiotherapy and concomitant and adjuvant temozolomide: translation of randomised controlled trial evidence into routine clinical practice. Clin Oncol- UK. 2011; 23: 372–373. [CrossRef]
- Gupta A, Young RJ, Karimi S, Sood S, Zhang Z, Mo Q, et al. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. Am J Neuroradiol. 2011; 32: 1301–1306. [CrossRef]
- Macdonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8: 1277–1280. [CrossRef]
- Shankar JJS, Bata A, Ritchie K, Hebb A, Walling S. Normalized Apparent Diffusion Coefficient in the Prognostication of Patients with Glioblastoma Multiforme. Can J Neurol Sci J Can Sci Neurol. 2016; 43: 127–133. [CrossRef]
- Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr. 2004; 28: 735–746. [CrossRef]
- Chenevert TL, Sundgren PC, Ross BD. Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimaging Clin N Am. 2006; 16: 619–632. [CrossRef]
- Gauvain KM, McKinstry RC, Mukherjee P, Perry A, Neil JJ, Kaufman BA, et al. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. Am J Roentgenol. 2001; 177: 449–454. [CrossRef]
- El Kady RM, Choudhary AK, Tappouni R. Accuracy of apparent diffusion coefficient value measurement on PACS workstation: A comparative analysis. Am J Roentgenol. 2011; 196: W280-W284. [CrossRef]
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010; 28: 1963–1972. [CrossRef]
- Clarke B, Schmidt MH, Pickett GE. P.043 Presence of infiltrative glioblastoma cells in an isolated area of diffusion restriction. Can J Neurol Sci. 2017; 44: S24–S25. [CrossRef]
- Clarke JL, Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009; 9: 241–246. [CrossRef]


