Regulation of Inflammatory Response in Islet Transplantation
Yoshitaro Shindo![]() , Mazhar A. Kanak *
, Mazhar A. Kanak *![]()
- Department of Surgery, School of Medicine, Virginia Commonwealth University, Richmond, USA
* Correspondence: Mazhar A. Kanak![]()
Received: March 29, 2018 | Accepted: June 11, 2018 | Published: June 25, 2018
OBM Transplantation 2018, Volume 2, Issue 2 doi:10.21926/obm.transplant.1802013
Academic Editor: Pål Dag Line
Special Issue: Current Advancement of Islet Cell Transplantation in the Treatment of Diabetes Mellitus
Recommended citation: Shindo Y, Kanak MA. Regulation of Inflammatory Response in Islet Transplantation. OBM Transplantation 2018;2(2):013; doi:10.21926/obm.transplant.1802013.
© 2018 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Islet cell transplantation is a developing treatment for patients suffering from severe Type-1 diabetes. The long-term insulin independence after islet cell transplantation has been difficult to achieve, and this has been linked to several factors. One of the major cause of poor long-term outcome is inflammation surrounding the islets. Inflammation in islets is caused at several stages, donor induced, during organ preservation, islet isolation stress, peri-transplant inflammation or instant blood mediated inflammatory reaction (IBMIR), and post-transplant hypoxia. In addition to inflammation, auto/allo-immune attack causes rejection and the toxicity of the immunosuppressive agents used can also affect islet transplant outcomes. In this review, we will summarize the various inflammatory processes that occur during islet transplantation along with past and previous approaches used to reduce inflammation in pre-clinical and clinical studies.
Keywords
Islet; inflammation; transplantation; hypoxia; rejection
1. Introduction
Pancreatic islet transplantation for severe type 1 diabetes (T1D) patients incorporates islet cells which confers euglycemia and reduces the life-threatening hypoglycaemic episodes. T1D is caused due to autoimmune destruction of pancreatic beta cells that produce insulin required for maintaining glucose homeostasis. Although insulin administration can somewhat manage the disease, its long term complications has been overwhelming. Alternatively, islet transplantation can provide tight glycemic control and prevent long-term complications. In 2000, the Edmonton group reported insulin independence in 7 consecutive islet transplant recipients treated with a glucocorticoid-free immunosuppressive regimen for 1 year [1]. However, insulin-independence rates have declined to 10% after 5 years, due to poor long-term graft survival [2]. The phase 3 clinical trial for transplantation of human islets to T1D patients with severe hypoglycaemic episodes reported <7% Hba1C in 70% of patients after 2 years and hypoglycaemic awareness was restored. The study concluded by recommending islet cell transplantation as a favourable option for T1D patients with severe hypoglycaemic unawareness [3]. However, rate of achievement of normoglycemia was <30% for 2 years, which is affected by several factors including islet isolation stress, peri-transplant inflammation, hypoxia, auto/allo-immune assault, and toxicity of immunosuppressive agents. In this review, we will discuss the mechanism and regulation of inflammatory response in islet transplantation and the factors that affect islet graft function (Figure 1).
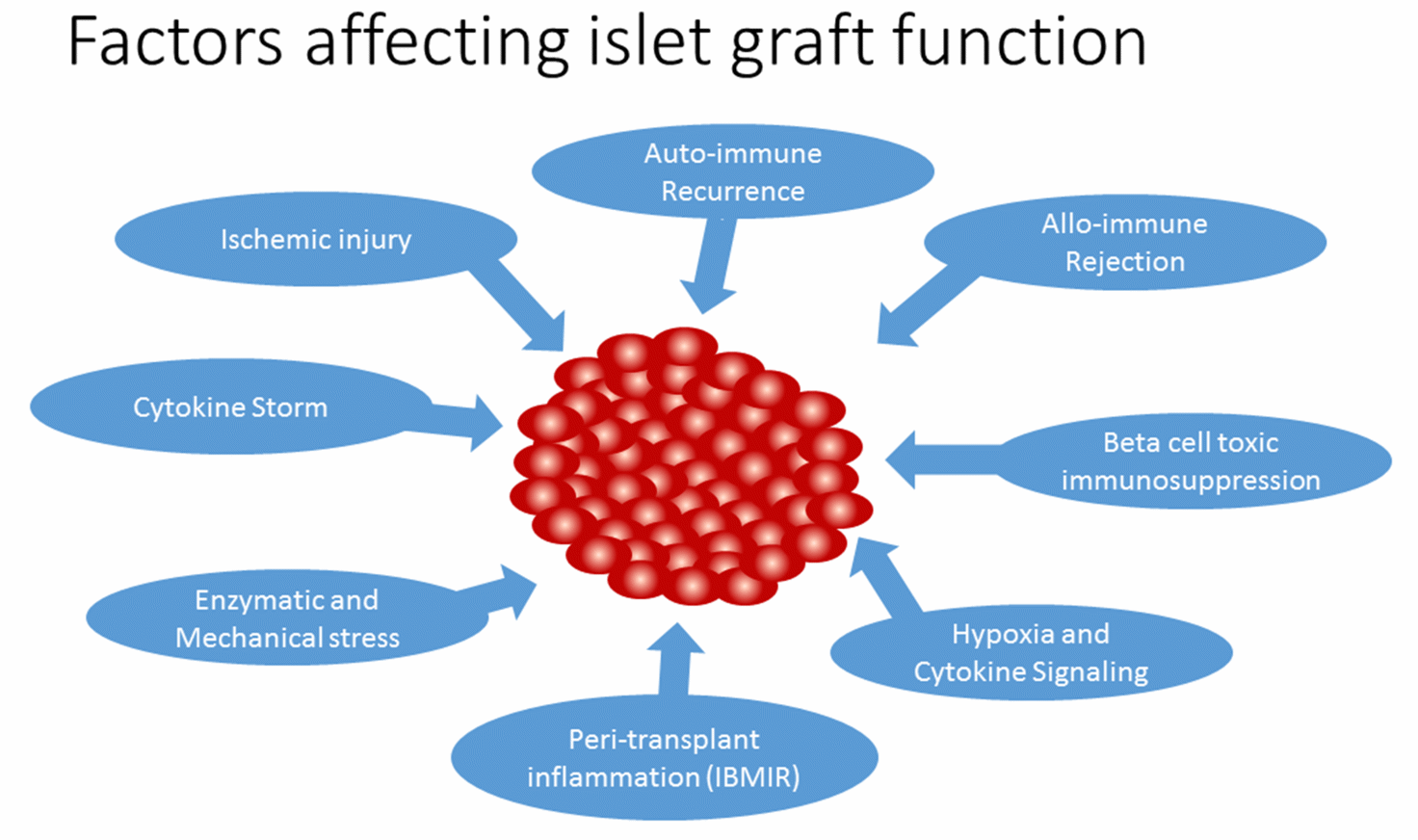
Figure 1 Factors affecting islet graft function.
2. Donor Induced Inflammation
2.1 Ischemia
Pancreas procurement initiates ischemia [4,5] and cold ischemic time (CIT) beyond 8 hours results in reduced islet yields [6], poor purification recovery [7] and graft dysfunction in human islet transplantation [8,9]. Cold ischemia time is not of major concern in autologous islet transplantation, as most centers have islet isolation facility for processing the pancreas. However this is an issue in allogeneic islet transplantation sometimes, because the organs are procured in a different hospital and has to be shipped to an islet isolation facility for processing which adds significant cold ischemic time. Pancreas persufflation through both the superior mesenteric artery and either the splenic artery (human) or celiac trunk (pig) demonstrated increase in ATP levels which was beneficial to human and porcine islets [10]. Preservation strategies to reduce ischemic injury needs to be further explored.
2.2 Cytokine Storm
Brain-dead condition leads to “cytokine storm”, which causes systemic inflammation and deteriorates islet function [11,12]. In brain-dead rat model, proinflammatory cytokines such as IL-1β, TNF-α and IL-6 were increased in the serum and pancreas, which caused poor islet yield, and function in vitro and in vivo. Islets also overexpressed nuclear factor-kappa B (NF-κB) p50, c-Jun and ATF-2 [13]. Another study showed that brain-dead in combination with warm-ischemic stress increased expression of TF and MCP-1, which are inflammatory mediators of IBMIR [14].
Pre-treatment of brain-dead donors with IL-1 receptor antagonist (IL-1ra) increased engraftment and islet graft function by preventing innate immune activation and decreasing the expression of inflammatory cytokines/chemokines [15]. Another study reported that non-heart-beating donors yielded 12.6% more islets than brain-dead donors with comparable viability [16]. Short term outcome results of islet transplantation from DCD and DBD donors have been comparable, however, defined criteria for acceptance of DCD pancreas for islet isolation is necessary [17].
3. Inflammation Caused During Isolation
3.1 Enzymatic and Mechanical Stress
Enzymatic and mechanical stress during isolation contributes to early islet graft failure by inducing apoptosis/necrosis and production of proinflammatory cytokines [18,19]. The isolation procedure activates mitogen-activated protein kinase (MAPK) p38 and c-Jun NH2-terminal kinase (JNK), which leads to apoptosis in islets [18]. Gene expression analysis revealed upregulation of 4560 genes associated with chemokine, hypoxia, apoptosis and stress in the isolated islets [20]. Moreover, the top 15 upregulated genes have NFκB binding sites within their promotor region, suggesting that NFκB may be activated during isolation [21]. Additionally, isolated islets showed downregulation of anti-inflammatory IL-10 gene and upregulation of proinflammatory IL-8 gene [21,22]. Therefore, inhibiting the NF-κB pathway during isolation might improve islet survival and function.
4. Peri-Transplant Inflammation – IBMIR
Islets, which are commonly transplanted into the portal vein, are subjected to inflammatory reaction known as instant blood-mediated inflammatory response (IBMIR), which is characterized by coagulation, complement activation, and proinflammatory cytokine secretion by the islet infiltrating immune cells [23,24]. It is estimated that approximately 50% of transplanted islets are damaged by IBMIR during the peri-transplant period. This response occurs within hours to days and has been demonstrated in autologous, allogeneic and xenogeneic conditions [25,26,27]. Islet isolation and stress causes upregulation of proinflammatory chemokines MCP-1 and IL-8 by islets that upon contact with blood recruits macrophages and neutrophils into the islets. Islets also express high levels of TF that activates coagulation. All these events are common in autologous, allogeneic and xenogeneic islet transplant setting. Additionally in allogeneic and xenogeneic islets, there is binding of IgM antibodies to the islets which triggers the activation of complement pathway. A combination of coagulation, inflammatory cell infiltration and complement activation comprises the devastating effect of IBMIR [28].
4.1 Coagulation
Transplanted islets can activate coagulation cascade by tissue factor (TF) expressed on the surface of isolated islets. TF activates platelets, which is enhanced by direct binding to collagen or endothelial cells in transplanted islets [29,30]. Thrombin also activate platelets by binding to protease activated receptors (PARs) on their cell surface, which generates a clot capsule containing platelets and infiltrating CD11b+ leukocytes [31,32]. Preventing coagulation by thrombin inhibitor Melagatran, low molecular weight dextran sulfate, anti-TF antibody (CNTO859) and heparin suppresses IBMIR and improves islet function [31,33,34,35]. Recent study showed that Developmental endothelial locus-1 (Del-1) could regulate IBMIR by regulating coagulation activation and inflammation [36]. CD39 has been beneficial to exert anti-coagulant and anti-inflammatory properties [37].
4.2 Complement
Complement activation has also been reported in allo- and xenogeneic, but interestingly, not in autologous islet transplantation [27]. Activation of both classical and alternative pathways has been associated during IBMIR [38]. Specifically in xenogeneic islet transplantation, alternative complement pathways play a major role during IBMIR [39]. Therefore complement inhibition therapies such as Factor H [39], compstatin [40], soluble complement receptor 1 (CR1) and C5a-blocking peptide were developed and prevented IBMIR and improved islet function [41,42]. A recent study showed that pre-treatment of islets with APT070: a membrane-localizing C3 convertase inhibitor, protected human islets allograft from complement-mediated damage by reducing intra-islet inflammation and decreasing cytokine expression [43].
4.3 Innate Cell Infiltration and Inflammatory Cytokine Secretion
Innate immune cells in the islet microenvironment secrete IL-1β, which increases local expression of chemokines leading to inflammatory cell recruitment to islets [28,44,45,46]. In addition, IL-1β inhibits β cell function and promotes Fas-triggered apoptosis through NFκB activation [47]. Islets damaged during isolation release danger signals and cytokines such as high-mobility group box 1 (HMGB1), IL-1β, TNF-α, etc. [48,49,50,51,52]. HMGB1 can bind to receptor for advanced glycation end-products (RAGE) or Toll-like receptor (TLR) expressed on islets and immune cells and upregulate proinflammatory cytokines by the activation of transcription factors including NF-κB, MAPK, and Janus kinase-signal transducer and activator of transcription (JAK-STAT) [53,54,55,56,57,58]. It has been reported that downregulating HMGB1 activity improved islet survival and function [59,60,61].
Islets also secrete a variety of chemokines which recruit innate immune cells through receptors such as CXCR1 and CXCR2 [62,63,64,65]. Reparixin, an inhibitor of CXCR1/2, has shown improvement in islet transplantation by inhibiting the recruitment of polymorphonuclear leukocytes and NKT cells to the liver [62]. A phase II study have shown that patients receiving allogeneic islet transplant with reparixin showed improved safety and efficacy, which indicates decreased insulin requirement and positive C-peptide [62]. Another study showed that blocking NFκB in islets using Withaferin A prevents pro-inflammatory cytokine induced apoptosis and improves islet engraftment by alleviating IBMIR [24,66].
5. Hypoxia and Cytokine Signalling
5.1 Revascularization
Transplanted islets are dependent on oxygen diffusion from the surrounding microenvironment for their survival and function before the vasculature is formed [4]. It takes several days to weeks for the islets to re-establish blood flow [67,68]. However, islets remain in low oxygen tension even after several months [4,69,70]. Although vascular endothelial growth factor (VEGF) is known to modulate islet angiogenesis after transplantation, the expression of VEGF in transplanted islets is significantly reduced [71,72,73]. Recent study reported that islets treated with human embryonic stem cell-derived MSC overexpressing VEGF demonstrated improved islet graft revascularization, function and survival [74].
Alternative transplantation sites have been proposed to improve islet engraftment and function [75,76]. Subcutaneous space can be considered to be a suitable site for accessibility and spaciousness although it is limited by poor vascularization [75,76,77]. Prevascularization of the subcutaneous site has been reported to reduce islets loss and improved long-term outcomes of islet transplantation by inducing vascularization [77].
5.2 Hypoxia Induced Inflammation
Hypoxia produces reactive oxygen species (ROS) in and around transplanted islets [78]. Because β cells contain low levels of antioxidative enzymes such as catalase, superoxide dismutase and glutathione peroxidase, they are prone to oxidative stress associated with ROS [79,80,81,82]. Overexpression of antioxidants such as manganese superoxide dismutase and metallothionein in islets can protect β cells from oxidative stress [83,84]. Moreover, intra-ductal glutamine administration reduced oxidative stress and improved islet yield and graft function [85]. Recent studies have reported that systemic administration of antioxidants such as catalytic antioxidant redox modulator and exendin-4 improved islet survival and function by reducing oxidative stress [86,87].
6. Immunosuppression Mediated Islet Damage
Islet transplantation depends on potent immunosuppressive therapy to prevent immune graft rejection. However, immunosuppressive regimen is associated with islet toxicity and increased risk of malignancies and opportunistic infections [88]. Currently, the common immunosuppressive protocols used in clinical islet transplantation include induction immunosuppressive therapy with either ATG or IL-2 receptor monoclonal antibody, followed by maintenance immunosuppressive therapy including tacrolimus and sirolimus [89,90]. Tacrolimus induces β-cell apoptosis and inhibits insulin secretion [91,92]. The β-cell toxicity of sirolimus is controversial. Sirolimus has been reported to reduce insulin secretion, impair revascularization, and antagonize angiogenesis, which results in reduced islet engraftment [93,94]. On the other hand, sirolimus showed no negative effects in islet engraftment and proliferation [95,96].
Immune tolerance of the transplanted islets is much needed to eliminate the use of β-cell toxic immunosuppressive agents. Alternative strategies such as immunomodulatory molecules have been introduced to prevent graft rejection. Inhibition of CD40/CD154 costimulation pathway improved long-term islet allograft survival in non-human primates without immunosuppression [97]. However, the human clinical trial of a humanized anti-CD40L monoclonal antibody was suspended due to increased incidence of thromboembolic complications [98]. Another costimulatory blockade agent cytotoxic T-lymphocyte associated protein 4-immunoglobulin (CTLA4-Ig), which binds to CD80 and CD86 and inhibits T cell activation, demonstrated insulin independence from a single donor islet transplantation and prolonged allograft survival without calcineurin inhibitors [99].
7. Auto/Allo-Immune Rejection
7.1 Alloimmune Response
Multiple islet infusion are high risk factors for increased de novo DSA, which is associated with graft failure [100,101]. A recent study reported that 5/16 islet transplant recipients (31%) developed de novo DSA against mismatched donor HLA: three recipients post-first transplant; two recipients post-second transplant [102]. They demonstrated that de novo DSA was associated with decreased function at 3 months and graft loss at 12 months, due to antibody-mediated rejection [102]. However, alemtuzumab induction was linked with reduced incidence of subsequent DSA formation.
Another study showed that only 2 recipients (4.8%) among 42 islet transplant recipients had pre-formed anti-HLA antibodies [103]. They also demonstrated that 13/42 recipients (30.9%) developed de novo DSA against mismatched donor HLA, including one patients who had pre-formed DSA because of a previous kidney transplant. Surprisingly, there were no significant correlation between appearance of DSA and graft dysfunction. It is suggested that the reasons might relate to the low DSA titres, inability to activate the complement and the lack of allogenic endothelial targets in islet graft [103]. Alloimmune rejection remains a significant obstacle of successful islet transplant and further study will be required to characterize optimal patient-donor matching characteristics [101].
7.2 Autoimmune Recurrence
Four major islet autoantigens, glutamic acid decarboxylase 65 (GAD65), proinsulin, islet tyrosine phosphatase (IA-2), and zinc transporter 8 (ZnT8), have been identified and are targeted by autoreactive lymphocytes in T1D patients [104,105,106].
T1D patient’s undergoing islet transplantation still have autoimmune memory which has the capacity to destroy islets. Besides, immunosuppressive protocols to prevent rejection don’t affect autoimmune memory, which result in autoimmune recurrence [107,108].
It has been reported that autoreactive memory CD4+ and CD8+ T cells play major roles in the autoimmune recurrence after transplantation [109,110,111,112]. Islet specific memory T cells are preserved in the circulation for a long time and reactivated by islet antigen exposure during islet transplantation, which leads to rejection of transplanted islets. Compared to naïve T cells, memory T cells have higher sensitivity to antigenic stimulation and less dependent on costimulatory signals [113,114]. Recurrence of islet autoimmunity can happen regardless of HLA matching. It has been reported that GAD65-specific memory CD4+ T cells in the peripheral blood and pancreas transplant lymph nodes of T1D patients with recurrent autoimmunity expressed the identical TCR Vβ and CDR3 sequence [110,111]. In murine models, autoreactive CD4+ T cells specific for islet specific antigens (BDC-2.5 TCR transgenic cells) can destroy islet allografts, which were established in NOD SCID recipients, in the absence of donor MHC class II expression [115]. In another NOD mice model, islet β cell cytotoxicity assay demonstrated that activated macrophages induced by CD4+ T cells directly killed islet [116]. However, it remains to be elucidated that how HLA-restricted autoreactive CD8+ T cells can recognize and destroy allogenic islet grafts expressing disparate HLA molecules. In addition, lymphopenia caused by immunosuppression can induce homeostatic proliferation of autoreactive memory T cells, accompanied with increased production of IL-7 and IL-15 [108,117].
8. Current Anti-Inflammatory Clinical Trials
Several ongoing clinical trials including anti-inflammatory, immunosuppression and transplant site protocols are shown in Table 1. TNF-α is released during IBMIR and induces apoptosis in β cells. In addition, islets are also exposed to IL-1β during intraportal transplantation which can cause apoptosis. Anti TNF blockade with etanercept has been reported to improve islet engraftment in clinical trial on single-donor and marginal dose islet transplantation [118]. Previously, a combination therapy of Anakinra and Etanercept, in clinical islet transplantation showed that all patients achieved insulin independence from a single-donor islet transplantation [119]. These results might suggest synergistic effects to improve islet transplantation by regulating circulating inflammatory mediators such as IP-10, TNF-α and IL-1β [120].
Table 1 Ongoing clinical trials in islet transplantation
| Trial Number | Phase | Sponsor/investigator | Description |
|
NCT02520076 |
1/2 |
University of Alberta/Shapiro et al. |
To demonstrate AAT efficacy in preventing non-immunologic loss of transplanted islet mass in a single donor islet transplant |
|
NCT02505893 |
2 |
Ospedale San Raffaele/Piemonti et al. |
To investigate combination therapy consisting of minimal islet transplantation (1500 IEQ/kg BW), ATG, rapamycin and pegfilgastrim in T1D |
|
NCT02464878 |
2 |
Massachusetts General Hospital/Markmann et al. |
To evaluate AAT efficacy in achieving insulin independence after first infusion of single donor islets |
|
NCT02402439 |
1 |
University of California/Posselt et al. |
To test the safety and efficacy of gastrointestinal submucosal islet transplantation in T1D patients with kidney allografts |
|
NCT01909245 |
2 |
City of Hope Medical Center/Kandeel et al. |
To test the safety and efficacy of islet transplantation using ATG in T1D patients |
|
NCT02213003 |
1/2 |
University of Miami/Alejandro et al. |
To test the safety and efficacy of islet transplantation onto the omentum in T1D patients |
|
NCT00306098 |
2 |
University of Miami/Alejandro et al. |
To assess the effect and ability of infliximab, etanercept and exenatide in T1D patients by slet transplantation |
|
NCT01967888 |
2/3 |
Dompé Farmaceutici S.p.A/Bellin et al. |
To assess the safety and efficacy of reparixin (CXCR1/2 inhibitor) to improve transplant outcomes in TPIAT |
|
NCT03073577 |
1 |
University of Alberta/Shapiro et al. |
To evaluate the safety and cytoprotective capacity of antiaging glycopeptide (PKX-001) in islet transplantaion for T1D |
|
NCT01897688 |
3 |
Northwestern University/Luo et al. |
To demonstrate the safety and efficacy of islt transplanation under alemtuzumab induction for T1D |
|
NCT02727608 |
2 |
Uppsala University/Tufveson et al. |
To evaluate the safety and efficacy of eculizumab (complement inhibitor) to prevent destruction of islet grafts in T1D |
|
NCT01722682 |
1/2 |
Ospedale San Raffaele/Piemonti et al. |
To evaluate the safety and efficacy of bone marrow as site for islet transplantation in T1D patients |
|
NCT02064309 |
1/2 |
Uppsala University Hospital |
To investigate the safety of implantation of the human islet containing device Beta-Air in TiD |
|
NCT02713997 |
4 |
University of Minnesota/Bellin et. al. |
To investigate the use of etanercept with AAT to improve islet autograft survival and function in TPIAT patients |
9. Other Strategies to Prevent Inflammation
Encapsulation of islets have been investigated to protect against inflammatory and immune reactivity. A PTFE semipermeable membrane has been utilized to protect Islets from immune attack and thereby prevent allogenic rejection [121]. Microencapsulation of islets with gel based materials such as alginate or agarose has been used to prevent infiltration of immune cells, but poor angiogenesis, hypoxia and poor diffusion of insulin and have limited the use these materials [122]. Another drawback of micro and macro capsules is the need for an appropriate transplant site due to the increased graft volume [123]. Coating of the islet surface with a nanofilm of hydrogel material has the potential for reducing or eliminating the use of immunosuppression while addressing most of the problems faced by macro- and micro-encapsulation [124].
Another strategy of mesenchymal stem cells (MSCs), non-hematopoietic, multipotent and self-renewing cells, has been an attractive therapy in islet transplantation [125,126]. MSCs have been shown to reduce inflammatory responses of allogeneic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9 [127]. MSCs can also inhibit apoptosis and promote angiogenesis by secreting cytokines such as VEGF, hepatocyte growth factor (HGF) and transforming growth factor beta 1 (TGFβ1) [128,129,130,131]. Furthermore, MSCs have been demonstrated to differentiate into insulin producing cells [132,133]. In a phase I study, Wang et.al., has shown safety and efficacy of co-transplantation of autologous bone marrow-derived MSCs with islets in CP patients undergoing TPAIT [134]. MSC patients showed improved islet engraftment compared with controls, although the number of MSCs recipients were limited [134].
10. Conclusions
Inflammation in islet transplantation has been associated with poor outcomes in islet cell transplantation. Regulating inflammation at various stages of transplantation may promote long-term islet graft survival. A multi-centre approach is needed to develop strong anti-inflammatory strategies and implement in clinical islet transplantation for improved success. Future directions to improve the success of islet transplantation should focus on blocking the effect of IBMIR using novel more potent anti-inflammatory therapeutics. Furthermore, attack by immune cells needs to be addressed using improved micro or macro encapsulation strategies. Finally, development of alternative sources of islets from stem cells that can be altered by genetic tools such as CRISPR/Cas9 to prevent immune assault will be needed.
Author Contributions
Authors Yoshitaro Shindo and Mazhar A. Kanak were both involved in the literature compilation, writing, editing, proofreading from the beginning to end of the manuscript development. Mazhar A. Kanak was responsible for drafting the subtitles for the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
References
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New Engl J Med. 2000; 343: 230-238. [CrossRef]
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005; 54: 2060-2069. [CrossRef]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016; 39: 1230-1240. [CrossRef]
- Lau J, Henriksnas J, Svensson J, Carlsson PO. Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant. 2009; 14: 688-693. [CrossRef]
- Garcia-Contreras M, Tamayo-Garcia A, Pappan KL, Michelotti GA, Stabler CL, Ricordi C, et al. Metabolomics study of the effects of inflammation, hypoxia, and high glucose on isolated human pancreatic islets. J Proteome Res. 2017; 16: 2294-2306. [CrossRef]
- Leeser DB, Bingaman AW, Poliakova L, Shi Q, Gage F, Bartlett ST, et al. Pulsatile pump perfusion of pancreata before human islet cell isolation. Transplant Proc. 2004; 36: 1050-1051. [CrossRef]
- Lyon J, Manning Fox JE, Spigelman AF, Kim R, Smith N, O'Gorman D, et al. Research-focused isolation of human islets from donors with and without diabetes at the alberta diabetes institute isletCore. Endocrinology. 2016; 157: 560-569. [CrossRef]
- Caballero-Corbalan J, Brandhorst H, Malm H, Felldin M, Foss A, Salmela K, et al. Using HTK for prolonged pancreas preservation prior to human islet isolation. J Surg Res. 2012; 175: 163-168. [CrossRef]
- Berkova Z, Saudek F, Girman P, Zacharovova K, Kriz J, Fabryova E, et al. Combining donor characteristics with immunohistological data improves the prediction of islet isolation success. J Diabetes Res. 2016; 2016. DOI: 10.1155/2016/4214328 [CrossRef]
- Scott WE, 3rd, Weegman BP, Ferrer-Fabrega J, Stein SA, Anazawa T, Kirchner VA, et al. Pancreas oxygen persufflation increases ATP levels as shown by nuclear magnetic resonance. Transplant Proc. 2010; 42: 2011-2015. [CrossRef]
- Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008; 29: 603-630. [CrossRef]
- Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesth Scand. 2009; 53: 425-435. [CrossRef]
- Contreras JL, Eckstein C, Smyth CA, Sellers MT, Vilatoba M, Bilbao G, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003; 52: 2935-2942. [CrossRef]
- Saito Y, Goto M, Maya K, Ogawa N, Fujimori K, Kurokawa Y, et al. Brain death in combination with warm ischemic stress during isolation procedures induces the expression of crucial inflammatory mediators in the isolated islets. Cell Transplant. 2010; 19: 775-782. [CrossRef]
- Danobeitia JS, Hanson MS, Chlebeck P, Park E, Sperger JM, Schwarznau A, et al. Donor pretreatment with IL-1 receptor antagonist attenuates inflammation and improves functional potency in islets from brain-dead nonhuman primates. Cell Transplant. 2015; 24: 1863-1877. [CrossRef]
- Zhao M, Muiesan P, Amiel SA, Srinivasan P, Asare-Anane H, Fairbanks L, et al. Human islets derived from donors after cardiac death are fully biofunctional. Am J Transplant. 2007; 7: 2318-2325. [CrossRef]
- Andres A, Kin T, O'Gorman D, Livingstone S, Bigam D, Kneteman N, et al. Clinical islet isolation and transplantation outcomes with deceased cardiac death donors are similar to neurological determination of death donors. Transplant Int. 2016; 29: 34-40. [CrossRef]
- Abdelli S, Ansite J, Roduit R, Borsello T, Matsumoto I, Sawada T, et al. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004; 53: 2815-2823. [CrossRef]
- Chou FC, Sytwu HK. Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. J Biomed Sci. 2009; 16: 71. [CrossRef]
- Marselli L, Thorne J, Ahn YB, Omer A, Sgroi DC, Libermann T, et al. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J Clin Endocr Meta. 2008; 93: 1046-1053. [CrossRef]
- Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. Plos One. 2012; 7: e30415. [CrossRef]
- Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-κB activity: a role for p50. Clin Exp Immunol. 2004; 135: 64-73. [CrossRef]
- Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999; 48: 1907-1914. [CrossRef]
- Kanak MA, Takita M, Itoh T, SoRelle JA, Murali S, Kunnathodi F, et al. Alleviation of instant blood-mediated inflammatory reaction in autologous conditions through treatment of human islets with NF-κB inhibitors. Transplantation. 2014; 98: 578-584. [CrossRef]
- Monti P, Vignali D, Piemonti L. Monitoring inflammation, humoral and cell-mediated immunity in pancreas and islet transplants. Curr Diabetes Rev. 2015; 11: 135-143. [CrossRef]
- Nayak DK, Saravanan PB, Bansal S, Naziruddin B, Mohanakumar T. Autologous and allogenous antibodies in lung and islet cell transplantation. Front Immunol. 2016; 7: 650. [CrossRef]
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. 2014; 14: 428-437. [CrossRef]
- Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol. 2014;2014:451035. [CrossRef]
- Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004; 109: 2698-2704. [CrossRef]
- Shin JS, Kim JS, Kim JM, Jang JY, Kim YH, Kim HJ, et al. Minimizing immunosuppression in islet xenotransplantation. Immunotherapy. 2014; 6: 419-430. [CrossRef]
- Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002; 51: 1779-1784. [CrossRef]
- Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscl Throm Vas. 2004; 24: 1015-1022. [CrossRef]
- Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low-molecular weight dextran sulfate abrogates the instant blood-mediated inflammatory reaction induced by adult porcine islets both in vitro and in vivo. Transplant Proc. 2004; 36: 1186-1187. [CrossRef]
- Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, et al. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007; 84: 308-315. [CrossRef]
- Cabric S, Eich T, Sanchez J, Nilsson B, Korsgren O, Larsson R. A new method for incorporating functional heparin onto the surface of islets of langerhans. Tissue Eng Part C Methods. 2008; 14: 141-147. [CrossRef]
- Kourtzelis I, Kotlabova K, Lim JH, Mitroulis I, Ferreira A, Chen LS, et al. Developmental endothelial locus-1 modulates platelet-monocyte interactions and instant blood-mediated inflammatory reaction in islet transplantation. Thromb Haemostasi. 2016; 115: 781-788. [CrossRef]
- Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006; 82: 428-432. [CrossRef]
- Walpen AJ, Mohacsi P, Frey C, Roos A, Daha MR, Rieben R. Activation of complement pathways in xenotransplantation: an in vitro study. Transpl Immunol. 2002; 9: 271-280. [CrossRef]
- Kang HJ, Lee H, Ha JM, Lee JI, Shin JS, Kim KY, et al. The role of the alternative complement pathway in early graft loss after intraportal porcine islet xenotransplantation. Transplantation. 2014; 97: 999-1008. [CrossRef]
- van der Windt DJ, Marigliano M, He J, Votyakova TV, Echeverri GJ, Ekser B, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell Transplant. 2012; 21: 1791-1802. [CrossRef]
- Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, et al. Damage to porcine islets of langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000; 69: 711-719. [CrossRef]
- Tokodai K, Goto M, Inagaki A, Nakanishi W, Okada N, Okada H, et al. C5a-inhibitory peptide combined with gabexate mesilate prevents the instant blood-mediated inflammatory reaction in a rat model of islet transplantation. Transplant Proc. 2010; 42: 2102-2103. [CrossRef]
- Xiao F, Ma L, Zhao M, Smith RA, Huang G, Jones PM, et al. APT070 (mirococept), a membrane-localizing C3 convertase inhibitor, attenuates early human islet allograft damage in vitro and in vivo in a humanized mouse model. Brit J Pharmacol. 2016; 173: 575-587. [CrossRef]
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987; 79: 319-326. [CrossRef]
- Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of langerhans. Science. 1986; 232: 1545-1547. [CrossRef]
- Xenos ES, Stevens RB, Sutherland DE, Lokeh A, Ansite JD, Casanova D, et al. The role of nitric oxide in IL-1 beta-mediated dysfunction of rodent islets of langerhans. Implications for the function of intrahepatic islet grafts. Transplantation. 1994; 57: 1208-1212. [CrossRef]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002; 110: 851-860. [CrossRef]
- Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest. 1998; 102: 516-526. [CrossRef]
- Reffet S, Thivolet C. Immunology of pancreatic islet transplantation. Diabetes Metab. 2006; 32: 523-526. [CrossRef]
- Chhabra P, Wang K, Zeng Q, Jecmenica M, Langman L, Linden J, et al. Adenosine A(2A) agonist administration improves islet transplant outcome: evidence for the role of innate immunity in islet graft rejection. Cell Transplant. 2010; 19: 597-612. [CrossRef]
- Jiao ZZ, Li Y, Fan P, Guo J, Xue WJ, Ding XM, et al. 1,25(OH)2D3 prolongs islet graft survival by inflammatory inhibition. Transplant Proc. 2014; 46: 1615-1620. [CrossRef]
- Delaune V, Berney T, Lacotte S, Toso C. Intraportal islet transplantation: the impact of the liver microenvironment. Transplant Int. 2017; 30: 227-238. [CrossRef]
- Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008; 117: 3216-3226. [CrossRef]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004; 279: 7370-7377. [CrossRef]
- Kimura A, Naka T, Muta T, Takeuchi O, Akira S, Kawase I, et al. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. P Natl Acad Sci USA. 2005; 102: 17089-17094. [CrossRef]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009; 22: 240-273. [CrossRef]
- He Q, You H, Li XM, Liu TH, Wang P, Wang BE. HMGB1 promotes the synthesis of pro-IL-1beta and pro-IL-18 by activation of p38 MAPK and NF-kappaB through receptors for advanced glycation end-products in macrophages. Asian Pac J Cancer Prev. 2012; 13: 1365-1370. [CrossRef]
- Cheng Y, Xiong J, Chen Q, Xia J, Zhang Y, Yang X, et al. Hypoxia/reoxygenation-induced HMGB1 translocation and release promotes islet proinflammatory cytokine production and early islet graft failure through TLRs signaling. BBA-Mol Basis Dis. 2017; 1863: 354-364. [CrossRef]
- Kuraya D, Watanabe M, Koshizuka Y, Ogura M, Yoshida T, Asahi Y, et al. Efficacy of DHMEQ, a NF-kappaB inhibitor, in islet transplantation: I. HMGB1 suppression by DHMEQ prevents early islet graft damage. Transplantation. 2013; 96: 445-453. [CrossRef]
- Itoh T, Nitta T, Nishinakamura H, Kojima D, Mera T, Ono J, et al. HMGB1-mediated early loss of transplanted islets is prevented by anti-IL-6R antibody in mice. Pancreas. 2015; 44: 166-171. [CrossRef]
- Hwang YH, Kim MJ, Lee YK, Lee M, Lee DY. HMGB1 modulation in pancreatic islets using a cell-permeable A-box fragment. J Control Release. 2017; 246: 155-163. [CrossRef]
- Citro A, Cantarelli E, Maffi P, Nano R, Melzi R, Mercalli A, et al. CXCR1/2 inhibition enhances pancreatic islet survival after transplantation. J Clin Invest. 2012; 122: 3647-3651. [CrossRef]
- Cowley MJ, Weinberg A, Zammit NW, Walters SN, Hawthorne WJ, Loudovaris T, et al. Human islets express a marked proinflammatory molecular signature prior to transplantation. Cell Transplant. 2012; 21: 2063-2078. [CrossRef]
- Baumann B, Salem HH, Boehm BO. Anti-inflammatory therapy in type 1 diabetes. Curr Diabetes Rep. 2012; 12: 499-509. [CrossRef]
- Nano R, Racanicchi L, Melzi R, Mercalli A, Maffi P, Sordi V, et al. Human pancreatic islet preparations release HMGB1: (ir)relevance for graft engraftment. Cell Transplant. 2013; 22: 2175-2186. [CrossRef]
- SoRelle JA, Itoh T, Peng H, Kanak MA, Sugimoto K, Matsumoto S, et al. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013; 56: 814-824. [CrossRef]
- Brissova M, Powers AC. Revascularization of transplanted islets: can it be improved? Diabetes. 2008; 57: 2269-2271. [CrossRef]
- Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol. 2013; 2013. DOI: 10.1155/2013/352315 [CrossRef]
- Lau J, Carlsson PO. Low revascularization of human islets when experimentally transplanted into the liver. Transplantation. 2009; 87: 322-325. [CrossRef]
- Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011; 60: 2350-2353. [CrossRef]
- Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, et al. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003; 12: 627-635. [CrossRef]
- Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004; 53: 963-970. [CrossRef]
- Langlois A, Mura C, Bietiger W, Seyfritz E, Dollinger C, Peronet C, et al. In vitro and in vivo investigation of the angiogenic effects of liraglutide during islet transplantation. Plos One. 2016; 11: e0147068. [CrossRef]
- Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, Azadmanesh K, Habibi-Anbouhi M, Heremans Y, et al. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes. Sci Rep. 2015; 5: 9322. [CrossRef]
- Perez-Basterrechea M, Esteban MM, Alvarez-Viejo M, Fontanil T, Cal S, Sanchez Pitiot M, et al. Fibroblasts accelerate islet revascularization and improve long-term graft survival in a mouse model of subcutaneous islet transplantation. Plos One. 2017; 12: e0180695. [CrossRef]
- Sakata N, Aoki T, Yoshimatsu G, Tsuchiya H, Hata T, Katayose Y, et al. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes-Metab Res. 2014; 30: 1-10. [CrossRef]
- Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015; 33: 518-523. [CrossRef]
- Kim MJ, Lee Y, Jon S, Lee DY. PEGylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials. 2017; 133: 242-252. [CrossRef]
- Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Bio Med. 1996; 20: 463-466. [CrossRef]
- Kajikawa M, Fujimoto S, Tsuura Y, Mukai E, Takeda T, Hamamoto Y, et al. Ouabain suppresses glucose-induced mitochondrial ATP production and insulin release by generating reactive oxygen species in pancreatic islets. Diabetes. 2002; 51: 2522-2529. [CrossRef]
- Azevedo-Martins AK, Lortz S, Lenzen S, Curi R, Eizirik DL, Tiedge M. Improvement of the mitochondrial antioxidant defense status prevents cytokine-induced nuclear factor-kappaB activation in insulin-producing cells. Diabetes. 2003; 52: 93-101. [CrossRef]
- Mohseni Salehi Monfared SS, Larijani B, Abdollahi M. Islet transplantation and antioxidant management: a comprehensive review. World J Gastroentero. 2009; 15: 1153-1161. [CrossRef]
- Bertera S, Crawford ML, Alexander AM, Papworth GD, Watkins SC, Robbins PD, et al. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003; 52: 387-393. [CrossRef]
- Li X, Chen H, Epstein PN. Metallothionein protects islets from hypoxia and extends islet graft survival by scavenging most kinds of reactive oxygen species. J Biol Chem. 2004; 279: 765-771. [CrossRef]
- Avila J, Barbaro B, Gangemi A, Romagnoli T, Kuechle J, Hansen M, et al. Intra-ductal glutamine administration reduces oxidative injury during human pancreatic islet isolation. Am J Transplant. 2005; 5: 2830-2837. [CrossRef]
- Sklavos MM, Bertera S, Tse HM, Bottino R, He J, Beilke JN, et al. Redox modulation protects islets from transplant-related injury. Diabetes. 2010; 59: 1731-1738. [CrossRef]
- Padmasekar M, Lingwal N, Samikannu B, Chen C, Sauer H, Linn T. Exendin-4 protects hypoxic islets from oxidative stress and improves islet transplantation outcome. Endocrinology. 2013; 154: 1424-1433. [CrossRef]
- Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: More than a membrane story. World J Transplant. 2016; 6: 69-90. [CrossRef]
- Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012; 35: 1436-1445. [CrossRef]
- Kloster-Jensen K, Sahraoui A, Vethe NT, Korsgren O, Bergan S, Foss A, et al. Treatment with tacrolimus and sirolimus reveals no additional adverse effects on human islets in vitro compared to each drug alone but they are reduced by sdding glucocorticoids. J Diabetes Res. 2016; 2016. DOI: 10.1155/2016/4196460. [CrossRef]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006; 443: 345-349. [CrossRef]
- Rangel EB. Tacrolimus in pancreas transplant: a focus on toxicity, diabetogenic effect and drug-drug interactions. Expert Opin Drug M. 2014; 10: 1585-1605. [CrossRef]
- Trapp A, Weis M. The impact of immunosuppression on endothelial function. J Cardiovasc Pharm. 2005; 45: 81-87. [CrossRef]
- Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, et al. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006; 55: 2429-2436. [CrossRef]
- Gao R, Ustinov J, Korsgren O, Otonkoski T. Effects of immunosuppressive drugs on in vitro neogenesis of human islets: mycophenolate mofetil inhibits the proliferation of ductal cells. Am J Transplant. 2007; 7: 1021-1026. [CrossRef]
- Melzi R, Maffi P, Nano R, Sordi V, Mercalli A, Scavini M, et al. Rapamycin does not adversely affect intrahepatic islet engraftment in mice and improves early islet engraftment in humans. Islets. 2009; 1: 42-49. [CrossRef]
- Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. P Natl Acad Sci USA. 1999; 96: 8132-8137. [CrossRef]
- Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000; 6: 114. [CrossRef]
- Posselt AM, Szot GL, Frassetto LA, Masharani U, Tavakol M, Amin R, et al. Islet transplantation in type 1 diabetic patients using calcineurin inhibitor-free immunosuppressive protocols based on T-cell adhesion or costimulation blockade. Transplantation. 2010; 90: 1595-1601. [CrossRef]
- Mosaad YM. Clinical role of human leukocyte antigen in health and disease. Scand J Immunol. 2015; 82: 283-306. [CrossRef]
- Tatum JA, Meneveau MO, Brayman KL. Single-donor islet transplantation in type 1 diabetes: patient selection and special considerations. Diabetes Metab Syndr Obes. 2017; 10: 73-78. [CrossRef]
- Brooks AM, Carter V, Liew A, Marshall H, Aldibbiat A, Sheerin NS, et al. De novo donor-specific HLA antibodies are associated with rapid loss of graft function following islet transplantation in type 1 diabetes. Am J Transplant. 2015; 15: 3239-3246. [CrossRef]
- Pouliquen E, Baltzinger P, Lemle A, Chen CC, Parissiadis A, Borot S, et al. Anti-donor HLA antibody response after pancreatic islet grafting: characteristics, risk factors, and impact on graft function. Am J Transplant. 2017; 17: 462-473. [CrossRef]
- Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007; 148: 1-16. [CrossRef]
- Pietropaolo M, Towns R, Eisenbarth GS. Humoral autoimmunity in type 1 diabetes: prediction, significance, and detection of distinct disease subtypes. Csh Perspect Med. 2012; 2. DOI: 10.1101/cshperspect.a012831. [CrossRef]
- Wenzlau JM, Hutton JC. Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diabetes Rep. 2013; 13: 608-615. [CrossRef]
- Stegall MD, Lafferty KJ, Kam I, Gill RG. Evidence of recurrent autoimmunity in human allogeneic islet transplantation. Transplantation. 1996; 61: 1272-1274. [CrossRef]
- Pugliese A, Reijonen HK, Nepom J, Burke GW, 3rd. Recurrence of autoimmunity in pancreas transplant patients: research update. Diabetes Management. 2011; 1: 229-238. [CrossRef]
- Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, et al. Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. P Natl Acad Sci USA. 2005; 102: 18425-18430. [CrossRef]
- Laughlin E, Burke G, Pugliese A, Falk B, Nepom G. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol. 2008; 128: 23-30. [CrossRef]
- Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, et al. Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes. 2010; 59: 947-957. [CrossRef]
- Newby BN, Mathews CE. Type I interferon is a catastrophic feature of the diabetic islet microenvironment. Front Endocrinol. 2017; 8: 232. [CrossRef]
- Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002; 106: 127-138. [CrossRef]
- Kumar R, Ferez M, Swamy M, Arechaga I, Rejas MT, Valpuesta JM, et al. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 2011; 35: 375-387. [CrossRef]
- Kupfer TM, Crawford ML, Pham K, Gill RG. MHC-mismatched islet allografts are vulnerable to autoimmune recognition in vivo. J Immunol. 2005; 175: 2309-2316. [CrossRef]
- Calderon B, Suri A, Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am J Pathol. 2006; 169: 2137-2147. [CrossRef]
- Monti P, Heninger AK, Bonifacio E. Differentiation, expansion, and homeostasis of autoreactive T cells in type 1 diabetes mellitus. Curr Diabetes Rep. 2009; 9: 113-118. [CrossRef]
- Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. Jama. 2005; 293: 830-835. [CrossRef]
- Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1beta and TNF-alpha. Cell Transplant. 2011; 20: 1641-1647. [CrossRef]
- Yoshimatsu G, Kunnathodi F, Saravanan PB, Shahbazov R, Chang C, Darden CM, et al. Pancreatic beta-cell-derived IP-10/CXCL10 isletokine mediates early loss of graft function in islet cell transplantation. Diabetes. 2017; 66: 2857-2867. [CrossRef]
- De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997; 63: 824-830. [CrossRef]
- Arifin DR, Manek S, Call E, Arepally A, Bulte JW. Microcapsules with intrinsic barium radiopacity for immunoprotection and X-ray/CT imaging of pancreatic islet cells. Biomaterials. 2012; 33: 4681-4689. [CrossRef]
- de Vos P, Spasojevic M, Faas MM. Treatment of diabetes with encapsulated islets. Adv Exp Med Biol. 2010; 670: 38-53. [CrossRef]
- Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, et al. Device design and materials optimization of conformal coating for islets of langerhans. P Natl Acad Sci USA. 2014; 111: 10514-10519. [CrossRef]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968; 6: 230-247. [CrossRef]
- English K. Mesenchymal stem cells to promote islet transplant survival. Curr Opin Organ Tran. 2016; 21: 568-573. [CrossRef]
- Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009; 58: 1797-1806. [CrossRef]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109: 1292-1298. [CrossRef]
- Aksu AE, Horibe E, Sacks J, Ikeguchi R, Breitinger J, Scozio M, et al. Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol. 2008; 127: 348-358. [CrossRef]
- Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A, et al. Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun. 2009; 32: 33-42. [CrossRef]
- Hirabaru M, Kuroki T, Adachi T, Kitasato A, Ono S, Tanaka T, et al. A method for performing islet transplantation using tissue-engineered sheets of islets and mesenchymal stem cells. Tissue Eng Part C Methods. 2015; 21: 1205-1215. [CrossRef]
- Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004; 53: 1721-1732. [CrossRef]
- Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cell. 2007; 25: 2837-2844. [CrossRef]
- Wang H, Strange C, Nietert PJ, Wang J, Turnbull TL, Cloud C, et al. Autologous mesenchymal stem cell and islet cotransplantation: safety and efficacy. Stem Cell Transl Med. 2018. DOI: 10.1002/sctm.17-0139. [CrossRef]



