The Concerted Action of Multiple Mechanisms to Induce and Sustain Transplant Tolerance
Sylvaine You 1, 2, 3, ‡, * ![]() , Lucienne Chatenoud 1, 2, 3
, Lucienne Chatenoud 1, 2, 3 ![]()
- University of Paris Descartes, Sorbonne Paris Cité, 75015 Paris, France
- INSERM U1151, Institut Necker-Enfants Malades, 75015 Paris, France
- CNRS UMR 8253, Institut Necker-Enfants Malades, 75015 Paris, France
‡ Current Affiliation: INSERM U1016, Institut Cochin, 75014 Paris, France.
* Correspondence: Sylvaine You ![]()
Academic Editor: Jean Kwun
Received: September 25, 2018 | Accepted: October 30, 2018 | Published: November 09, 2018
OBM Transplantation 2018, Volume 2, Issue 4 doi:10.21926/obm.transplant.1804025
Special Issue: Multiple Aspects of Transplant Tolerance – Mechanisms, Strategies, and Barriers
Recommended citation: You S, Chatenoud L. The Concerted Action of Multiple Mechanisms to Induce and Sustain Transplant Tolerance. OBM Transplantation 2018; 2(4): 025; doi:10.21926/obm.transplant.1804025.
© 2018 by the authors. This is an open access article distributed under the conditions of the Creative Commons by Attribution License, which permits unrestricted use, distribution, and reproduction in any medium or format, provided the original work is correctly cited.
Abstract
Transplant tolerance has been achieved in experimental models using immune intervention strategies. Yet, their clinical translation remains unsuccessful and requires further optimization of immunotherapeutic regimens based on a deeper understanding of the cellular and molecular mechanisms at play in the induction and maintenance phases of immune tolerance. Intensive investigations have shed light on the tolerogenic networks underlying graft survival and have unraveled their complexity, which may depend on several parameters such as intrinsic features of the transplant itself (organs, tissues, and cells), the type of immunotherapy, and the therapeutic window used. It is now fairly well-established that Foxp3+ regulatory T lymphocytes (Tregs) play a central role in these networks. However, a wealth of evidence points to multiples mechanisms, including alloreactive T cell depletion and T cell anergy, that engage and cooperate in a timely manner in addition to Treg-mediated suppression to tip the balance from rejection towards robust and sustained tolerance. All of this evidence supports the fact that the clinical development of tolerance-inducing therapies may reside in the triggering of these multiple pathways to achieve long-term graft survival in transplanted patients while minimizing or withdrawing immunosuppression.
Keywords
Transplant tolerance; immune-intervention; regulatory T lymphocytes; anergy; T cell apoptosis; PD-1/PD-L1
1. Introduction
Chronic immunosuppressive regimens are necessary to protect transplanted cells, tissues, and organs from rejection. These treatments successfully prevent acute rejection episodes, however, they are accompanied by severe side- effects (infections, cancer, cardio-vascularcardiovascular diseases, etc) that, associatedin association with the intrinsic toxicity of the drugs, put a significant burden on patientthe patient's life and can lead to graft loss. Thus, despite the efforts developed to minimize generalized immunosuppression, more distinctly categorize the patients, and provide a more personalized treatment, alternative therapies are needed to improve long-term outcomes and reduce morbidity and mortality. Advances in the understanding of the genetic, cellular, and molecular mechanisms sustaining immune system homeostasis and regulation have encouraged the development of immuno-intervention strategies promoting robust antigen-specific tolerance without impeding effective responses to tumor antigens or infectious agents in transplanted recipients.
CD4+ regulatory T lymphocytes (Tregs) constitutively expressing the transcription factor Foxp3 (forkhead box P3), in addition to well-described markers such as CD25 (IL-2Rα chain), CTLA-4 (cytotoxic T lymphocyte-associated protein 4), GITRhigh (glucocorticoid-induced TNFR family related protein), CD127low (IL-7Rα chain), and FR4 (folate receptor 4), play a central role in the maintenance of immunological self-tolerance and also in the control of a broad spectrum of immune responses to infectious, transplant, and tumor antigens [1,2,3]. Regulatory T cells are heterogeneous in many ways: their origin (e.g., thymic versus peripheral, delineated by expression of neuropilin-1), their immune status (e.g., naïve, activated, or memory), their mode of action (e.g., production of immunomodulatory cytokines such as transforming growth factor β, interleukin-10, or interleukin-35; down-modulation of antigen-presenting cell functions through CTLA-4 or LAG3; cytolysis mediated by the secretion of cytotoxic molecules such as granzyme B and perforin; or metabolic perturbations linked to interleukin-2 or ATP deprivation), and also their antigen specificity [4,5,6]. In addition to antigen recognition, signals through accessory molecules and cytokines control their activation, expansion, and survival and regulate their suppressive capacities. In transplantation, Tregs can confer protection against graft rejection in various rodent or non-human primate models by controlling the alloimmune response in an antigen-specific manner [7,8,9]. Therefore, over the past few decades, a large body of preclinical studies concentrated on the propensity of therapeutic agents to upregulate the number and/or the functional capacity of these Tregs and thereby establish immunoregulatory mechanisms operative in vivo over the long term. This was particularly successful with monoclonal antibodies (Abs) targeting components of the antigen recognition and T cell activation pathways (CD3/T Cell receptor complex, CD4/CD8 co-receptors, major histocompatibility complex molecules, and co-stimulatory molecules). From these extensive investigations emerged the concept that Foxp3+ Tregs (thymus or peripherally-derived) are mandatory for the continuous and sustained antigen-specific control of alloreactive T cells, in particular within the graft microenvironment, through mechanisms of linked suppression and infectious tolerance [10,11,12].
However, we and others have demonstrated that transplantation tolerance relies on the concerted action of several tolerogenic mechanisms rather than on a single one. The induction and maintenance phases of tolerance may engage distinct pathways, namely depletion of effector T cells, Treg-mediated suppression, and T cell anergy; the balance between these 3 mechanisms is dependent on the transplant context and the therapeutic approach used.
2. Depletion of Alloreactive T cells
The first objective of immune-intervention strategies tested is to selectively downregulate the alloreactive responses raised against the transplanted organs while maintaining immune competence.
Numerous studies using monoclonal antibodies reported that a transient and partial depletion of T cells appeared to be crucial for tolerance induction, notably in fully mismatched conditions. Importantly, T cell subsets are not all equally sensitive to depletion therapy, which may be dictated by their nature, their immunological state, and the associated intensity of expression of the target molecules. Experimental data using anti-CD3, -CD4, -TCR, -CD45RB, and –CD45RC Abs showed a preferential targeting of effector T cells involved in the immune responses, leading to their apoptosis [13,14,15,16,17,18]. For instance, using a fully MHC-mismatched islet allograft model, we showed by single cell multiplex PCR that CD3 antibody F(ab’)2 fragments selectively eliminated graft-infiltrating CD8+ T cells co-expressing the cytolytic molecules granzyme B and perforin as well as CD4+ T cells co-expressing granzyme B and the transcription factor T-bet (which is the master regulator of Th1 responses), i.e., the main actors of islet destruction [14,19]. This depletion implicated the Fas/Fas ligand pathway. Interestingly, apoptosis of alloreactive T cells was also observed after a co-stimulatory blockade with CD40 ligand antibodies and/or the fusion protein CTLA-4Ig [20,21,22,23,24]. In these models, blockade of T cell apoptosis resulted in graft rejection instead of tolerance [20,21]. In the same manner, conditional and specific depletion of dividing alloreactive T cells expressing a suicide gene was sufficient to induce tolerance towards vascularized hearts or islet allografts [25,26].
Recently, M. Sykes and colleagues reported clonal deletion of donor-reactive T cells in a small cohort of tolerant recipients of combined kidney and bone marrow transplantation (CKBMT) [27]. In this sophisticated study, the authors used high-throughput next-generation sequencing of the TCR β chain CDR3 region to characterize and track alloreactive T cells in the blood before and after CKBMT. Donor-specific CD4+ and CD8+ T cell clone sizes (identified pretransplant by their proliferation in a mixed lymphocyte reaction) were found reduced in three tolerant patients (which were off immunosuppression for more than 4 years). This was not observed in one nontolerant CKBMT recipient who received the same therapeutic protocol but rejected his graft, or in two conventional renal allograft recipients on standard immunosuppressive regimens. Although limited by the small number of patients, this study suggests a role for clonal deletion in kidney allograft tolerance.
Taken collectively, these experimental and clinical data support depletion of alloreactive effector T cells as a central primary event for achieving transplant tolerance of MHC-mismatched organs. Reduction of the destructive T cell pool enables a reset of an immunoregulatory environment in which other mechanisms of immune tolerance, such as active suppression and anergy, can control the otherwise anti-donor response.
3. Relative Role of Foxp3 Tregs for the Acquisition and the Maintenance of Tolerance
A number of investigations demonstrated that the same monoclonal Abs that efficiently eliminate or inhibit effector T cells actually spare Foxp3+ Tregs, in contrast [13,14,15,16,17,18]. Similar observations were reported after treatment with anti-lymphocyte serum or anti-thymocyte globulin [28,29,30,31,32]. Consequently, Treg frequency (but not necessarily absolute numbers) increases in lymphoid organs and in the grafts, thus creating a local Treg-enriched microenvironment amenable to the induction of immune tolerance, and referring to an “immune privileged site” [33].
The critical involvement of Foxp3+ Tregs in transplant tolerance stems from the multiple studies showing the preferential accumulation of Tregs within the grafted organs after immunotherapy and the occurrence of graft rejection in recipients deprived of Tregs at the time of transplantation and treatment. However, few studies addressed the question of the permanent requirement of Tregs during both the induction and the maintenance phase of tolerance. Different outcomes were reported according to the grafted organ and the therapeutic strategy used (Tables 1 and Table 2). For instance, using a Foxp3hCD2 transgenic mouse model, Kendal et al. showed that permanent acceptance of a fully-mismatched skin graft by non-depleting CD4, CD8, and CD40 ligand antibodies was dependent on the constant presence of Tregs, notably of therapy-induced peripheral Tregs that infiltrated the grafted skin where they afforded a long-lasting and active control of effector T cells [12]. Similarly, administration of diphtheria toxin (DT) to Foxp3DTR recipients of kidney allografts three weeks or three months after transplantation induced acute rejection with massive CD4+ and CD8+ T cell infiltration in the renal cortex [34]. In another setting, early or late administration of the anti-CD25 antibody (PC61) abrogated long-term cardiac or islet allograft survival induced by a combined treatment with rapamycin and trichostatin A, a histone deacetylase inhibitor [35], and by the use of streptavidin-Fas ligand-engineered pancreatic islets and rapamycin [36], respectively.
In contrast, a series of reports showed that although Foxp3+ Tregs play a key role to induce transplant tolerance, they are dispensable to maintain it over the long-term (Table 2). Indeed, their removal at the time of transplantation and/or treatment precipitated graft rejection, but their late elimination by PC61, at a distance from transplantation and therapy once the tolerance was established, was without effect on graft survival and function. This was observed in different transplant contexts using skin, pancreatic islets, heart, liver or hematopoietic stem cells (HSC) from minor-mismatched or fully-mismatched donors. It is true that these data should be considered in light of the limitation to complete depletion of target cells (70-80%), and we cannot exclude that access of long-term tolerated grafts to the inhibitory effects of PC61 may not be equivalent in the inductive and maintenance phases of tolerance. However, the repeated failure of this antibody to reverse long-established tolerance strongly argues for the existence of complementary mechanisms, which become more dominant in the long term. We confirmed this point by using Foxp3DTR C57BL/6 mice grafted under the kidney capsule with pancreatic islets from BALB/c mice and treated with CD3 Abs. Administration of DT, which induced a profound Treg depletion (>95%) on day 100 post-transplant, had no impact on islet survival [14]. In the same manner, islet allograft recipient mice rendered tolerant after therapy with anti-CD45RB Ab, rapamycin, and interleukin (IL)-10 did not reject their islet allograft after treatment with P60, a peptide inhibitor of Foxp3 that downregulates Foxp3 nuclear translocation and abrogates Treg-suppressive capacities [37,38]. Finally, in our islet transplant model, administration of an antibody targeting CTLA-4, which was expressed quasi-exclusively by Foxp3+ Tregs and mandatory for their regulatory functions, provided the same results as PC61. Abrogation of tolerance induction by CD3 Abs (which resulted in graft rejection when injected at the time of therapy) but not maintenance (which resulted in graft survival when injected on day 100 after transplantation) was observed [14].
Table 1 Requirement of Foxp3+ Tregs for induction and maintenance of transplant tolerance

Other types of T cells exhibiting regulatory properties can take over and be critical for sustaining long-term graft survival. For instance, in the context of anti-CD45RB Ab, rapamycin, and IL-10 combination therapy, Gagliani et al. showed that Foxp3+ Tregs and de novo-generated T regulatory type 1 (Tr1) cells played non-redundant roles, the former being key for the induction of tolerance and the latter for its maintenance [17,38]. In addition, Tregs exerted their suppressive function locally within the islet allograft, while Tr1 cells acted from the spleen in an antigen- and IL-10-dependent manner. This suggested a transfer of tolerance from the graft-infiltrating Foxp3+ Tregs to splenic Tr1 cells [38] in accordance with the concept of infectious tolerance proposed by Herman Waldmann [10,40]. The key role of Tr1 cells was possibly attributed to the therapeutic regimen using IL-10, as it has not been reported so far in other settings. In our model, CD3 Ab therapy was still efficacious at inducing long-term islet survival in IL-10-/- recipients (Figure 1). Notably, identical results were obtained in IL-4-/- mice, excluding a prominent role of Th2 cells in CD3 Ab-induced tolerance (Figure 1).
Table 2 Requirement of Foxp3+ Tregs for induction but not maintenance of transplant tolerance


Figure 1 Transplant tolerance induced by CD3 Ab therapy does not depend on IL-10 or IL-4. Wild-type (n=12), IL-10-/- (n=7) or IL-4-/- (n=5) C57BL/6 mice were transplanted under the kidney capsule with BALB/c pancreatic islets and treated with CD3 Ab F(ab’)2 fragments (50µg/day i.v. for 5 consecutive days, starting on day 7 post-transplant, grey area). Blood glucose levels were measured for 110 days. Islet graft survival was similar between all groups.
4. T cell Anergy as a Mechanism to Sustain Long-Term Graft Survival
A sustained T cell unresponsiveness or anergy has been correlated with permanent allograft survival in different transplant settings. Anergy is defined as a hyporesponsive, non-proliferative state occurring when T cells recognize their cognate antigens (signal 1) in the absence of appropriate costimulation (signal 2), usually provided by CD28 on T cells interacting with CD80 or CD86 on antigen presenting cells [48]. In addition to signals 1 and 2, other molecules and pathways have an impact on T cell fate such as the engagement of inhibitory receptors (e.g., CTLA-4, programmed cell death-1, and T-cell immunoglobulin and mucin domain 3) and the production of immunosuppressive cytokines (such as IL-10 and transforming growth factor [TGF]Β)[49]. In transplantation, these mediators have been shown to play a central role in promoting allograft survival and immune tolerance [50,51,52,53].
T cell anergy to transplanted organs is revealed by the absence of proliferation and cytokine production towards the cognate alloantigens, while reactivity against third-party antigens or mitogens remains intact. This was notably demonstrated through TCR transgenic T cells used to track and characterize alloantigen-specific T cells in vivo in untreated and treated recipients [52,54,56,57,58]. After CD3 Ab therapy, we observed that intragraft T cells as well as peripheral purified CD8+ and Foxp3-CD4+ T cells were unable to mount anti-donor IFNγ responses over the long-term, supporting an intrinsic and sustained inability to react against donor antigens [19]. Multiplex PCR analysis of individual CD4+ and CD8+ effector T cells present within the graft on day 100 post-transplant further confirmed this unresponsive state by showing a complete lack of co-expression of cytotoxic or Th1 markers (granzyme B, Fas ligand, perforin, or T-bet), thus highlighting an absence of effector and cytolytic capacities [14,19]. In contrast, single cell analysis revealed TGFβ mRNA expression by a majority of graft-infiltrating T cells (both CD4+ and CD8+) after CD3 Ab therapy, and TGFβ signaling within T cells was mandatory to induce T cell anergy and graft survival [19]. Of interest, TGFβ positively controlled the expression of the inhibitory receptor programmed cell death (PD)-1, and even more strongly that of its ligand PD-L1 on T cells, thereby evidencing a direct link between these two regulatory pathways [19].
PD-1 and PD-L1 have been shown to play key roles in controlling T cell response to alloantigens and inducing and maintaining T cell unresponsiveness. PD-1 is inducibly expressed on T cells after TCR engagement while PD-L1 is characterized by its broad expression on hematopoietic and non-hematopoietic cells, including transplanted tissues, and is upregulated upon activation [51,59,60]. In vitro, PD-1-/- or PD-L1-/- T cells responded more vigorously than wild-type T cells to alloantigen stimulation in terms of activation, proliferation, and inflammatory cytokine production [61,62]. Similar results were obtained using allogeneic PD-L1-/- antigen presenting cells [61]. In vivo, abrogation of PD-L1 expression through genetic deficiency or neutralizing antibodies in the donor organ or in the transplant recipient accelerated graft rejection (Table 3, upper panel; [55,61,63]). In the same regard, PD-1 or PD-L1 blockade abrogated long-term cardiac allograft survival obtained in CD80/CD86 deficient mice [64] and reversed the spontaneous acceptance of liver transplants [65]. This was associated with the inhibition of alloreactive T cell apoptosis and anergy as shown by enhanced T cell activation, proliferation, and polarization towards an inflammatory Th1 phenotype. Of note, PD-L1 can also interact with CD80 expressed by antigen presenting cells (APCs), and active PD-L1/CD80 ligation has been associated with allograft survival [66,67,68].
We and others have demonstrated the key contribution the PD-1/PD-L1 pathway in transplant tolerance induced by various immunotherapeutic approaches (Table 3, lower panel). PD-1 upregulation on alloreactive CD4+ and CD8+ T cells is frequently associated with long-term graft survival after immune intervention therapy [14,19,56,62,69]. Of interest, in our islet transplant model, PD-1 was co-expressed with PD-L1 on a majority of CD8+ T cells present within the graft after CD3 Ab therapy and up to day 100 post-transplant. This observation suggested a paracrine interaction with the neighboring PD-1+ T cells leading to their mutual inhibition [19]. In different transplant settings, neutralizing PD-1 and/or PD-L1 signaling at the time of transplantation and treatment (by using specific antibodies or PD-1- or PD-L1-deficient hosts or organ donors) abrogated induction of tolerance by costimulatory blockers such as CD40L Abs or CTLA-4Ig, alone or in combination [52,56,58,62,70,71,72], or by CD3 Abs [19]. Of importance, a key role of the PD-1/PD-L1 pathway has been demonstrated not only in the induction phase but also in the maintenance phase of tolerance. Indeed, after tolerance was established by CD3 Ab therapy, we showed that administration of PD-L1 Abs on day 100 post-transplant precipitated islet graft rejection [19]. Similar results were reported in a cardiac allograft model where tolerance, induced by CTLA-4Ig therapy, was abrogated after PD-L1 neutralization on day 60 post-transplant [71]. Lastly, treatment with anti-PD-1 or anti-PD-L1 Abs led to graft loss in OT-I chimeric recipients of long-term surviving mOVA skin grafts of greater than 120 days [56]. Blockade of PD-1 and/or PD-L1 induced prolonged synapses between alloreactive T cells and APCs; it also induced reversal of the anergic state as revealed by the re-acquisition of proliferative potential as well as effector and cytotoxic functions, leading to graft destruction [56,61,62,65,71,73]. Altogether, these data clearly demonstrate the requirement of an active PD-1/PD-L1 signaling pathway for the effective and sustained dampening of allogeneic T cell responses to promote tolerance induction and maintenance.
Table 3 Inhibition of transplant tolerance after blockade of the PD-1/PD-L1 pathway

5. Conclusions
Multiple mechanisms, in particular in the fully MHC-mismatched context, cooperate in a timely manner to induce and maintain robust transplantation tolerance (Figure 2). Therapeutic protocols combining drugs allowing the complementary actions of alloreactive T cell depletion and unresponsiveness in combination with Treg-mediated suppression may thus be considered to achieve long-term graft survival while minimizing or withdrawing immunosuppression in transplanted patients. In this regard, triggering PD-1/PD-L1 signals may provide an attractive strategy. Indeed, it has been shown that engagement of PD-1 signaling on T cells after administration of a fusion protein (consisting of the extracellular domain of PD-L1 and the Fc portion of IgG synergized with anti-CD40L) blunted T cell alloreactivity and induced permanent islet allograft survival [75,76].
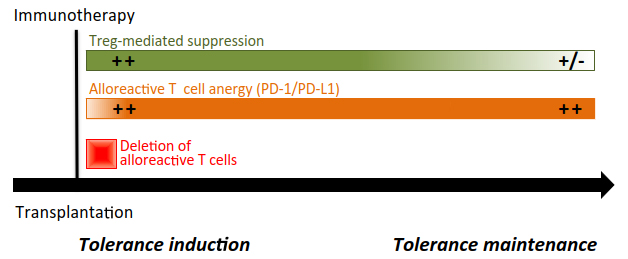
Figure 2 Transplant tolerance induction and maintenance. For most of immunotherapeutic approaches, induction of tolerance requires the depletion of allogeneic effector T cells (implicating the Fas/FasL pathway, in particular), the induction of anergy in the remaining alloreactive T cells through the expression and activation of the PD-1/PD-L1 and TGF-β/TGF-βR signalings, and active Foxp3+ Treg-mediated control (through CTLA-4 and TGF-β) of residual and reconstituting naïve T cells. Once established, this tolerogenic micro-environnement relies on the sustained intrinsic unresponsiveness of T cells towards the transplanted organ. Foxp3+ Tregs certainly contributes to maintain tolerance, but their active engagement may vary depending on the transplant context.
Acknowledgments
The authors would like to thank the doctoral and postdoctoral fellows who participated in our research work and in particular M. Bass, A. Besançon and D. Calderon. They are also indebted to T. Goncalves, F. Valette and S. Fonlebeck for the technical help and animal care.
Author Contributions
S.Y. wrote the original and the revised manuscript. L.C. provided critical advice and help in writing and reviewing the manuscript.
Funding
The laboratory of the authors was supported by institutional funding from INSERM, CNRS and Paris Descartes University and grants from the European Commission (FP6; RISET consortium: Reprogramming the Immune System for the Establishment of Tolerance), the Juvenile Diabetes Research Foundation (JDRF), Fondation CENTAURE and Fondation Day Solvay.
Competing Interests
The authors have declared that no competing interests exist.
References
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003; 299: 1057-1061. [CrossRef]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005; 22: 329-341. [CrossRef]
- Huynh A, Zhang R, Turka LA. Signals and pathways controlling regulatory T cells. Immunol Rev. 2014; 258: 117-131. [CrossRef]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012; 30: 531-564. [CrossRef]
- Pereira LMS, Gomes STM, Ishak R, Vallinoto ACR. Regulatory T cell and Forkhead Box Protein 3 as modulators of immune homeostasis. Front Immunol. 2017; 8: 605. [CrossRef]
- Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017; 17: 703-717. [CrossRef]
- Long E, Wood KJ. Regulatory T cells in transplantation: transferring mouse studies to the clinic. Transplantation. 2009; 88: 1050-1056. [CrossRef]
- Cobbold SP, Waldmann H. Regulatory cells and transplantation tolerance. Cold Spring Harb Perspect Med. 2013; 3: 671-674. [CrossRef]
- Waldmann H, Hilbrands R, Howie D, Cobbold S. Harnessing FOXP3+ regulatory T cells for transplantation tolerance. J Clin Invest. 2014; 124: 1439-1445. [CrossRef]
- Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science. 1993; 259: 974-977. [CrossRef]
- Waldmann H, Cobbold S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity. 2001; 14: 399-406. [CrossRef]
- Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011; 208: 2043-2053. [CrossRef]
- You S, Zuber J, Kuhn C, Baas M, Valette F, Sauvaget V, et al. Induction of allograft tolerance by monoclonal CD3 antibodies: a matter of timing. Am J Transplant. 2012; 12: 2909-2919. [CrossRef]
- Besancon A, Baas M, Goncalves T, Valette F, Waldmann H, Chatenoud L, et al. The induction and maintenance of transplant tolerance engages both regulatory and anergic CD4+ T cells. Front Immunol. 2017; 8: 218. [CrossRef]
- Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J Immunol. 2011; 187: 2015-2022. [CrossRef]
- Nagahama K, Fehervari Z, Oida T, Yamaguchi T, Ogawa O, Sakaguchi S. Differential control of allo-antigen-specific regulatory T cells and effector T cells by anti-CD4 and other agents in establishing transplantation tolerance. Int Immunol. 2009; 21: 379-391. [CrossRef]
- Gagliani N, Gregori S, Jofra T, Valle A, Stabilini A, Rothstein DM, et al. Rapamycin combined with anti-CD45RB mAb and IL-10 or with G-CSF induces tolerance in a stringent mouse model of islet transplantation. PLoS One. 2011; 6: e28434. [CrossRef]
- Picarda E, Bezie S, Boucault L, Autrusseau E, Kilens S, Meistermann D, et al. Transient antibody targeting of CD45RC induces transplant tolerance and potent antigen-specific regulatory T cells. JCI Insight. 2017; 2: e90088. [CrossRef]
- Baas M, Besancon A, Goncalves T, Valette F, Yagita H, Sawitzki B, et al. TGFbeta-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife. 2016; 5: e08133. [CrossRef]
- Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999; 5: 1298-1302. [CrossRef]
- Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999; 5: 1303-1307. [CrossRef]
- Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, Greiner DL. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000; 164: 512-521. [CrossRef]
- Verbinnen B, Billiau AD, Vermeiren J, Galicia G, Bullens DM, Boon L, et al. Contribution of regulatory T cells and effector T cell deletion in tolerance induction by costimulation blockade. J Immunol. 2008; 181: 1034-1042. [CrossRef]
- Monk NJ, Hargreaves RE, Marsh JE, Farrar CA, Sacks SH, Millrain M, et al. Fc-dependent depletion of activated T cells occurs through CD40L-specific antibody rather than costimulation blockade. Nat Med. 2003; 9: 1275-1280. [CrossRef]
- Braunberger E, Cohen JL, Boyer O, Pegaz-Fiornet B, Raynal-Raschilas N, Bruneval P, et al. T-Cell suicide gene therapy for organ transplantation: induction of long-lasting tolerance to allogeneic heart without generalized immunosuppression. Mol Ther. 2000; 2: 596-601. [CrossRef]
- Giraud S, Barrou B, Sebillaud S, Debre P, Klatzmann D, Thomas-Vaslin V. Transient depletion of dividing T lymphocytes in mice induces the emergence of regulatory T cells and dominant tolerance to islet allografts. Am J Transplant. 2008; 8: 942-953. [CrossRef]
- Morris H, DeWolf S, Robins H, Sprangers B, LoCascio SA, Shonts BA, et al. Tracking donor-reactive T cells: Evidence for clonal deletion in tolerant kidney transplant patients. Sci Transl Med. 2015; 7: 272ra10. [CrossRef]
- Minamimura K, Gao W, Maki T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol. 2006; 176: 4125-4132. [CrossRef]
- Valdez-Ortiz R, Bestard O, Llaudo I, Franquesa M, Cerezo G, Torras J, et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl Int. 2015; 28: 108-119. [CrossRef]
- Hire K, Ngo DK, Stewart-Maynard KM, Hering B, Bansal-Pakala P. FoxP3+, and not CD25+, T cells increase post-transplant in islet allotransplant recipients following anti-CD25+ rATG immunotherapy. Cell Immunol. 2012; 274: 83-88. [CrossRef]
- D’Addio F, Boenisch O, Magee CN, Yeung MY, Yuan X, Mfarrej B, et al. Prolonged, low-dose anti-thymocyte globulin, combined with CTLA4-Ig, promotes engraftment in a stringent transplant model. PLoS One. 2013; 8: e53797. [CrossRef]
- Buszko M, Cardini B, Oberhuber R, Oberhuber L, Jakic B, Beierfuss A, et al. Differential depletion of total T cells and regulatory T cells and prolonged allotransplant survival in CD3E humanized mice treated with polyclonal anti human thymocyte globulin. PLoS One. 2017; 12: e0173088. [CrossRef]
- Forrester JV, Xu H, Lambe T, Cornall R. Immune privilege or privileged immunity? Mucosal Immunol. 2008; 1: 372-381. [CrossRef]
- Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011; 178: 1635-1645. [CrossRef]
- Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007; 13: 1299-1307. [CrossRef]
- Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice. J Immunol. 2011; 187: 5901-5909. [CrossRef]
- Casares N, Rudilla F, Arribillaga L, Llopiz D, Riezu-Boj JI, Lozano T, et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J Immunol. 2010; 185: 5150-5159. [CrossRef]
- Gagliani N, Jofra T, Valle A, Stabilini A, Morsiani C, Gregori S, et al. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. Am J Transplant. 2013; 13: 1963-1975. [CrossRef]
- Yamazaki M, Pearson T, Brehm MA, Miller DM, Mangada JA, Markees TG, et al. Different mechanisms control peripheral and central tolerance in hematopoietic chimeric mice. Am J Transplant. 2007; 7: 1710-1721. [CrossRef]
- Cobbold SP, Adams E, Graca L, Daley S, Yates S, Paterson A, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006; 213: 239-255. [CrossRef]
- Jiang X, Morita M, Sugioka A, Harada M, Kojo S, Wakao H, et al. The importance of CD25+ CD4+ regulatory T cells in mouse hepatic allograft tolerance. Liver Transpl. 2006; 12: 1112-1118. [CrossRef]
- Weng L, Dyson J, Dazzi F. Low-intensity transplant regimens facilitate recruitment of donor-specific regulatory T cells that promote hematopoietic engraftment. Proc Natl Acad Sci U S A. 2007; 104: 8415-8420. [CrossRef]
- Perobelli SM, Mercadante AC, Galvani RG, Goncalves-Silva T, Alves AP, Pereira-Neves A, et al. G-CSF-induced suppressor IL-10+ neutrophils promote regulatory T cells that inhibit graft-versus-host disease in a long-lasting and specific way. J Immunol. 2016; 197: 3725-3734. [CrossRef]
- Jiang X, Sun W, Guo D, Cui Z, Zhu L, Lin L, et al. Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery. 2011; 149: 336-346. [CrossRef]
- Banuelos SJ, Markees TG, Phillips NE, Appel MC, Cuthbert A, Leif J, et al. Regulation of skin and islet allograft survival in mice treated with costimulation blockade is mediated by different CD4+ cell subsets and different mechanisms. Transplantation. 2004; 78: 660-667. [CrossRef]
- Miller ML, Daniels MD, Wang T, Chen J, Young J, Xu J, et al. Spontaneous restoration of transplantation tolerance after acute rejection. Nat Commun. 2015; 6: 7566. [CrossRef]
- Calderon D, Prot M, You S, Marquet C, Bellamy V, Bruneval P, et al. Control of immune response to allogeneic embryonic stem cells by CD3 antibody-mediated operational tolerance induction. Am J Transplant. 2016; 16: 454-467. [CrossRef]
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003; 21: 305-334. [CrossRef]
- Valdor R, Macian F. Induction and stability of the anergic phenotype in T cells. Semin Immunol. 2013;25(4):313-20. [CrossRef]
- Ford ML. T Cell Cosignaling Molecules in Transplantation. Immunity. 2016;44(5):1020-33. [CrossRef]
- Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012; 12: 2575-2587. [CrossRef]
- Lucas CL, Workman CJ, Beyaz S, LoCascio S, Zhao G, Vignali DA, et al. LAG-3, TGF-beta, and cell-intrinsic PD-1 inhibitory pathways contribute to CD8 but not CD4 T-cell tolerance induced by allogeneic BMT with anti-CD40L. Blood. 2011; 117: 5532-5540. [CrossRef]
- Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003; 4: 1093-1101. [CrossRef]
- Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005; 175: 771-779. [CrossRef]
- Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005; 174: 3408-3415. [CrossRef]
- Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, et al. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol. 2008; 181: 5313-5322. [CrossRef]
- Fehr T, Lucas CL, Kurtz J, Onoe T, Zhao G, Hogan T, et al. A CD8 T cell-intrinsic role for the calcineurin-NFAT pathway for tolerance induction in vivo. Blood. 2010; 115: 1280-1287. [CrossRef]
- Miller ML, Daniels MD, Wang T, Wang Y, Xu J, Yin D, et al. Tracking of TCR-transgenic T cells reveals that multiple mechanisms maintain cardiac transplant tolerance in mice. Am J Transplant. 2016; 16: 2854-2864. [CrossRef]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004; 101: 10691-10696. [CrossRef]
- Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008; 117: 660-669. [CrossRef]
- Wang W, Carper K, Malone F, Latchman Y, Perkins J, Fu Y, et al. PD-L1/PD-1 signal deficiency promotes allogeneic immune responses and accelerates heart allograft rejection. Transplantation. 2008; 86: 836-844. [CrossRef]
- Takahashi T, Hsiao HM, Tanaka S, Li W, Higashikubo R, Scozzi D, et al. PD-1 expression on CD8(+) T cells regulates their differentiation within lung allografts and is critical for tolerance induction. Am J Transplant. 2018; 18: 216-225. [CrossRef]
- Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006; 177: 5928-5935. [CrossRef]
- Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005; 174: 6648-6656. [CrossRef]
- Morita M, Fujino M, Jiang G, Kitazawa Y, Xie L, Azuma M, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant. 2010; 10: 40-46. [CrossRef]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007; 27: 111-122. [CrossRef]
- Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011; 187: 1113-1119. [CrossRef]
- Ni X, Song Q, Cassady K, Deng R, Jin H, Zhang M, et al. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest. 2017; 127: 1960-1977. [CrossRef]
- Truong W, Plester JC, Hancock WW, Merani S, Murphy TL, Murphy KM, et al. Combined coinhibitory and costimulatory modulation with anti-BTLA and CTLA4Ig facilitates tolerance in murine islet allografts. Am J Transplant. 2007; 7: 2663-2674. [CrossRef]
- Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007; 37: 2983-2990. [CrossRef]
- Tanaka K, Albin MJ, Yuan X, Yamaura K, Habicht A, Murayama T, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol. 2007; 179: 5204-5210. [CrossRef]
- Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008; 112: 2149-2155. [CrossRef]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009; 10: 1185-1192. [CrossRef]
- Miller ML, McIntosh CM, Williams JB, Wang Y, Hollinger MK, Isaad NJ, et al. Distinct graft-specific TCR avidity profiles during acute rejection and tolerance. Cell Rep. 2018; 24: 2112-2126. [CrossRef]
- Ozkaynak E, Wang L, Goodearl A, McDonald K, Qin S, O’Keefe T, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002; 169: 6546-6553. [CrossRef]
- Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation. 2003; 76: 994-999. [CrossRef]



